2012 was a Grand Cru year for the FDA with 39 NMEs approved
As we herald in a New Year, it is time to reflect a little on the past year. 2012 was, to paraphrase Professor Bertrand Tombal’s quote about prostate cancer drug development, “a Grand Cru year” for the United States Food & Drug Administration (FDA) with 39 new molecular entitites (NMEs) approved. This is the highest approval number in the last 10 years, beating the previous high of 36 obtained in 2004. Reuters report it is a 16 year high.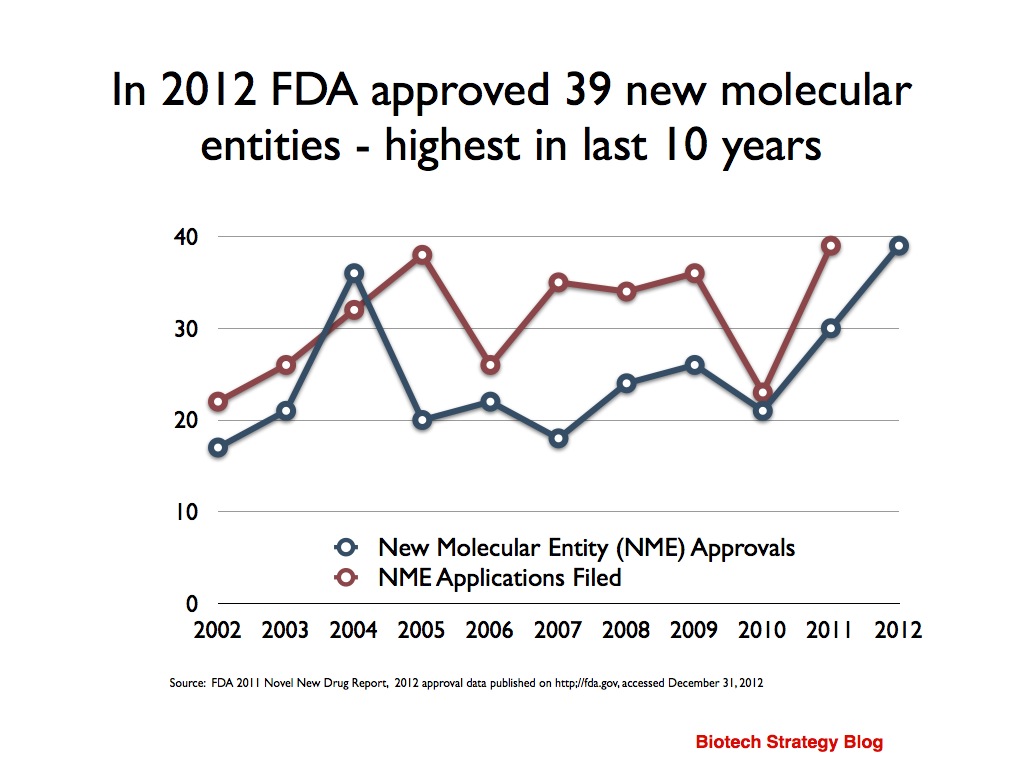
Unfortunately, I don’t think can we can draw many conclusions about the state of drug development innovation from this 2012 high.
The FDA in their 2011 report on novel new drugs note that “the number of NMEs approved over time has not been substantially increasing.”
To me, the overall picture looks pretty flat. There’s bound to be variation between years as a result of timing differences with some regulatory submissions obtaining priority review, while others do not. Some companies can take longer than others to close a clinical trial database and prepare a dossier. We also have to factor in that some clinical trials may end earlier than expected, if the data is positive.
Regulatory approval is the result of innovation that started several years ago. It only represents the point at which you have a safe and efficaceous product that can be sold to the public. The number of approvals in any given year is not a surrogate benchmark for the state of current innovation.
Given it typically takes several years to bring a new product to market, what we are looking at today is the result of research done 5-10 years ago. It is, however, interesting to note that of the 39 NME approvals in 2012, one-third (13) were cancer related.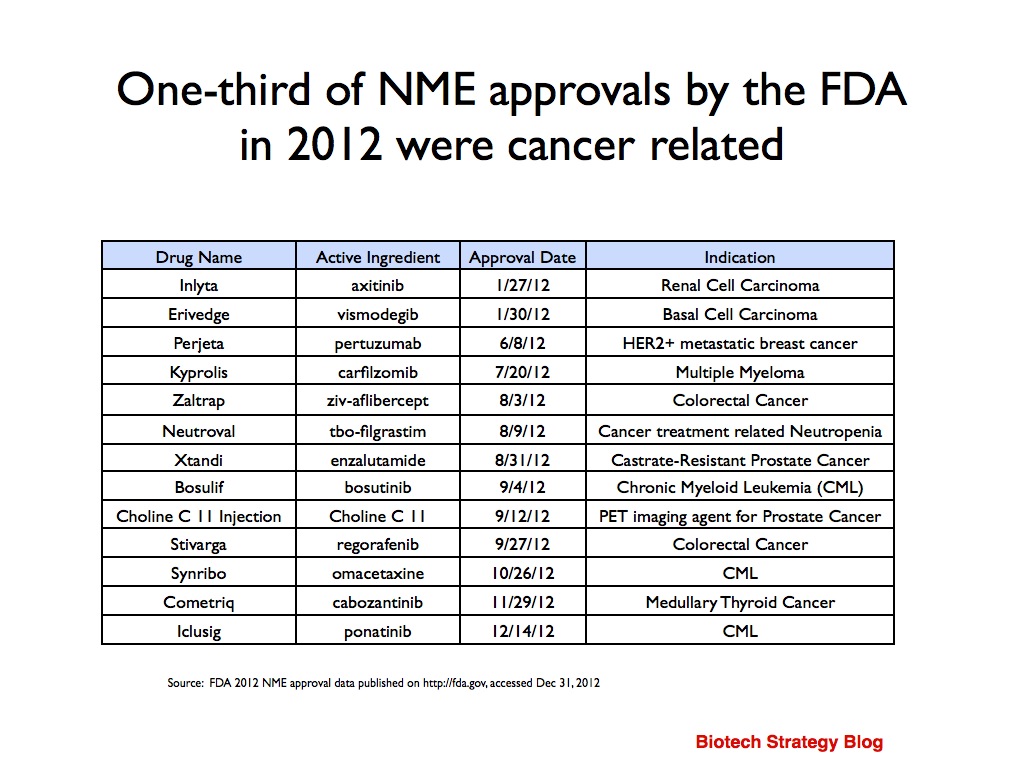
A key driver of innovation in this area is the increased knowledge we have of cancer biology.
In my view, investors will continue to support companies that develop new products with:
- a clear scientific rationale as to why their mechanism of action may impact the disease
- a focused clinical development plan that through use of biomarkers and diagnostics targets those most likely to respond
- a market opportunity worth going after in what is increasingly a competitive landscape
2012 was a “grand cru year” for the FDA. I look forward to what 2013 may bring and to learning more about the new products in development that may make a difference to the lives of patients.
Happy New Year!