PIVOT data continues to show no survival benefit for prostatectomy over watchful waiting in men with low to medium risk early prostate cancer
 Timothy J. Wilt MD, MPH presented an update on the VA, NCI, AHRQ Prostate cancer Intervention Versus Observation Trial (PIVOT) on the final day of the 2012 European Association of Urology (EAU) Congress in Paris.
Timothy J. Wilt MD, MPH presented an update on the VA, NCI, AHRQ Prostate cancer Intervention Versus Observation Trial (PIVOT) on the final day of the 2012 European Association of Urology (EAU) Congress in Paris.
I previously wrote on this blog about the PIVOT data presented by Professor Wilt in the plenary session at the 2011 American Urological Association Annual meeting.
The PIVOT trial objective according to Dr Wilt, was to answer the following question:
Among men with clinically localized prostate cancer detected during the early PSA era, does the intent to treat with radical prostectomy reduce all-cause & prostate cancer mortality compared to observation?
PIVOT enrolled 731 men from 1994 to 2002 who were randomized to either receive radical prostatectomy or undergo just observation.
The results from the trial provide level 1 evidence based medicine (highest standard) concerning the survival benefits conferred by radical prostactectomy (with the potential for quality of life impacts such as incontinence & erectile dysfunction), as compared to not undertaking surgery, but instead doing observation only in the form of watchful waiting or active monitoring.
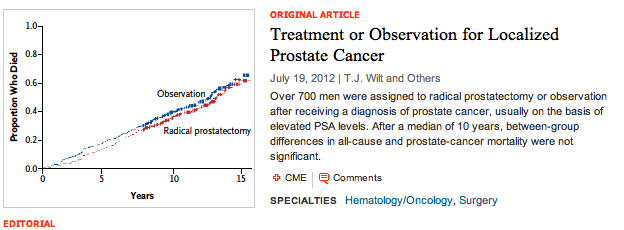
Dr Wilt told the urologists in the EAU 2012 Congress plenary session, that after a median follow-up of 10 years (interquartile range = 7.3 to 12.6), the median survival was 12.7 years. Wilt told the audience that:
“Prostate cancer mortality was uncommon occurring in only 7.1% of men, it did not vary considerably by patient age, race, comorbidities or health status, but did vary considerably by tumor risk status ranging from 3 % in men with low risk disease to 13 % in men with high risk disease.”
PIVOT Prostate Cancer Mortality Results
No of Deaths: 52/731 (7.1%)
- Low risk (3.4%)
- High risk (8.4%)
- High risk (13.3%)
In the men who had death judged to be due to prostate cancer, “absolute differences between treatments were less than 1%,” Wilt said.
As far as I could determine, the data presented at EAU 2012 was no different from the PIVOT data presented at AUA 2011 other than being another year mature.
A subgroup analysis showed that surgery conferred no survival benefit over watchful waiting except for high-risk patients. In his EAU 2012 presentation, Dr Wilt described the subgroup findings in more detail (emphasis added):
Low Risk Prostate Cancer
“In men with low risk prostate cancer, disease mortality occurred in less than 3% and did not differ between radical prostatectomy and observation” (HR=1.48; ARR=1.4, P=0.54). This favored observation.”
High Risk Prostate Cancer
“Among men with high risk tumors, prostate cancer mortality occurred in approximately 13%. Radical prostatectomy produced a 60% relative risk reduction (HR = 0.4, ARR = 8.4) of borderline significance (P=0.04).
Intermediate Risk Prostate Cancer
“Among men with intermediate risk prostate cancer, we found a non-significant reduction of 4.6%.”
PSA <= 10ng/ml
“In men with PSA <= 10ng/ml there was no significant difference between radical prostatectomy and watchful waiting.” (HR = 0.92, ARR=0.3%, P=0.82). The findings were virtually identical throughout the course of the study. The lines are essentially superimposable for prostate cancer mortality in men treated with observation or with radical prostatectomy.”
PSA > 10ng/ml
“Among men with baseline PSA > 10ng/ml, radical prostatectomy reduced prostate cancer death by a relative 64% and an absolute difference of 7.2%. You can see the curves begin to separate at approximately 7 years.” (HR=0.36, ARR= 7.2%, P=0.03)
 Dr Wilt’s conclusion based on the latest study data was that:
Dr Wilt’s conclusion based on the latest study data was that:
“In men with localized prostate cancer detected during the early PSA era, radical prostatectomy compared to observation did not significantly reduce all-cause and prostate cancer mortality. Absolute differences through at least 12 years were less than 3%”
These results are important findings that should impact the treatment of men diagnosed with early stage, low risk prostate cancer.
The fact that the survival curves do not diverge except for high-risk patients presenting with a PSA > 10ng/mL, may also have an impact on the ongoing PSA prostate screening debate.
If the PIVOT data results in more men being put on watchful waiting/active monitoring, then it should lower the overtreatment that screening currently produces. Urologists will, however, need to be prepared to counsel their patients accordingly and forego the economic benefits that undertaking surgery affords many of them.
Urologists at the EAU in Paris greeted the PIVOT trial data in silence and an absence of social media interaction (I did not see any urologists tweet enthusiastically about it).
Many urologists who have trained many years to perform complex surgical techniques may find the idea of watchful waiting an anathema.
Adopting a policy of watchful waiting in many prostate cancer patients may also place economic pressures placed on those urologists who need a throughput of patients to recover or amortize the cost of expensive technology such as the da Vinci robotic system.
The PIVOT trial data is, however, level 1 evidence based medicine that cannot be ignored.
Hopefully, this analysis of the PIVOT trial data will be published in a peer-reviewed journal in the not too distant future so that it can reach a wider audience than those urologists who attended the AUA 2011 and EAU 2012 plenary sessions.
Update July 18, 2012
The results of the PIVOT trial presented at AUA 2011 and EAU 2012 have been published in the New England Journal of Medicine (online first, July 18, 2012).
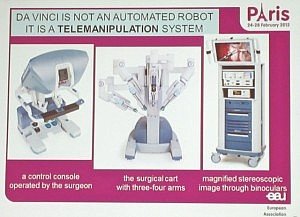 Something that I was not aware of until I attended the media briefing was that so called “robotic surgery” is not an automated robot performing the surgery on its own, but instead it’s actually robot assisted surgery.
Something that I was not aware of until I attended the media briefing was that so called “robotic surgery” is not an automated robot performing the surgery on its own, but instead it’s actually robot assisted surgery.


 There was a lot of interest in yesterday’s advanced prostate cancer poster session at EAU 2012.
There was a lot of interest in yesterday’s advanced prostate cancer poster session at EAU 2012. Orteronel (TAK-700) is a selective, non-steroidal inhibitor of 17, 20 lyase, a key enzyme involved in the production of androgens such as testosterone. This is a similar mode of action to
Orteronel (TAK-700) is a selective, non-steroidal inhibitor of 17, 20 lyase, a key enzyme involved in the production of androgens such as testosterone. This is a similar mode of action to 
 Today sees the start of the
Today sees the start of the  After the recent JP Morgan Healthcare conference, San Francisco remains the destination of choice for forthcoming medical meetings.
After the recent JP Morgan Healthcare conference, San Francisco remains the destination of choice for forthcoming medical meetings.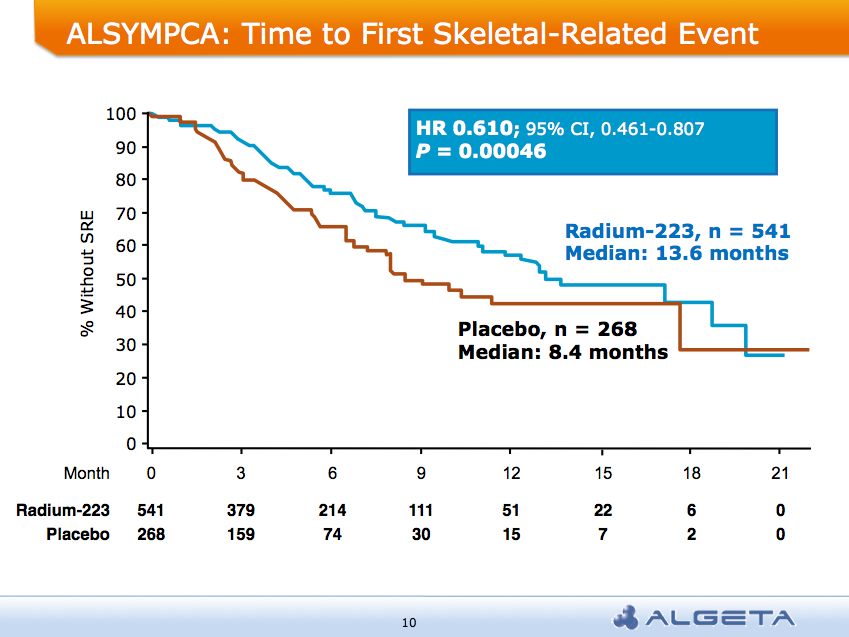 AND a median overall survival of 14 months compared to 11.2 months for placebo group:
AND a median overall survival of 14 months compared to 11.2 months for placebo group: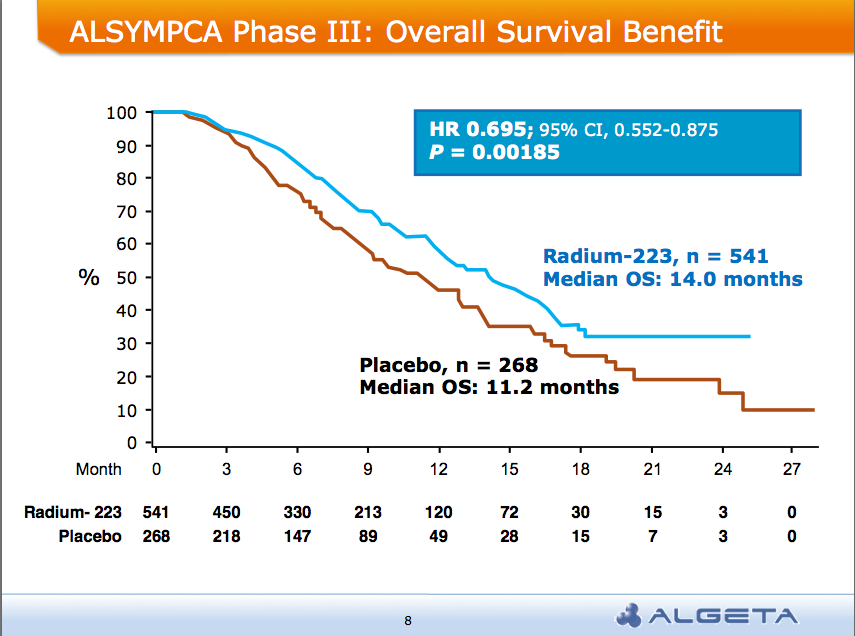 Alpharadin is on the fast track to FDA approval this year
Alpharadin is on the fast track to FDA approval this year

 The recent AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics international conference in San Francisco was an informative meeting.
The recent AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics international conference in San Francisco was an informative meeting. This morning the 8am session at the
This morning the 8am session at the  The highlight, in my opinion, was Oliver Sartor’s excellent presentation on radium-223 chloride (Alpharadin) in which he cogently outlined its mechanism of action. He explained that radium-223:
The highlight, in my opinion, was Oliver Sartor’s excellent presentation on radium-223 chloride (Alpharadin) in which he cogently outlined its mechanism of action. He explained that radium-223: Prostate cancer is the second leading cause of cancer death in men, so it was good news this morning when Medivation & Astellas issued a
Prostate cancer is the second leading cause of cancer death in men, so it was good news this morning when Medivation & Astellas issued a