AZD3514 Fails to Live up to Preclinical Promise in CRPC at ASCO 2013
AZD3514 is a novel Selective Androgen Receptor Down-Regulating Drug (SARD) that showed early preclinical promise for the treatment of Castration-Resistant Prostate Cancer (CRPC).
However the development of this drug in advanced prostate cancer has been terminated by AstraZeneca according to Dr Aurelius Omlin, a Clinical Research Fellow at The Royal Marsden Hospital who presented clinical data on AZD3514 at the 2013 annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago.
I previously wrote about the promising preclinical data for AZD3514 presented by Sarah Loddick at the 2012 annual meeting of the American Association for Cancer Research (AACR) and sometimes drugs when they transition to the clinic just don’t live up to their promise.
That’s what happened here, and it reminds us that testing of drugs on human volunteers remains a key part of drug development despite the inherent risks. (See my post on the TLS deaths on the AbbVie/Genentech ABT-199 CLL dose finding trial)
 The results from a first-in-human clinical trial with in men with CRPC were presented by Dr Omlin at ASCO 2013 (abstract 4511). In his oral presentation, he first noted that:
The results from a first-in-human clinical trial with in men with CRPC were presented by Dr Omlin at ASCO 2013 (abstract 4511). In his oral presentation, he first noted that:
“AZD3514 is a first-in-class, non-steroidal small molecule androgen receptor (AR) down-regulator that inhibits nuclear AR translocation and results in proteasomal AR protein degradation.”
The phase 1 clinical trial to assess safety and tolerability explored doses ranging from 100mg once daily (OD) to 1000mg OD in capsule formulation, and from 1000mg OD to 2000mg taken twice daily (BID) in tablet formulation. A pretty comprehensive range, but……
“Tolerability of AZD3514 was problematic,” said Omlin. “80% of patients had Grade 1-2 Nausea (n=39 out of 49) and 49% Grade 1-2 Vomiting (n=24 out of 49).” Additionally, grade 1-2 thrombocytopenia was seen in 33% of patients. There was no dose limiting toxicity reported.
What killed it for AZD3514 was the fact that according to Omlin,
“Nausea and vomiting were characteristic from the very first dose level starting about 30-60 minutes after dosing and lasting for several hours thereafter.”
However, the drug did show activity in CRPC patients with several patients showing PSA declines including one patient with prior abiraterone exposure. Two patients with soft tissue disease had confirmed responses according to Recist 1.1. There was also evidence of clinical activity from changes in the number of circulating tumor cells.
Industry analyst, David Miller (@BiotechStockRsr) commented on Twitter, while watching the presentation, that he thought it hard to see the drug progressing in development, and he turned out to be correct:
AZD-3514 $AZN had huge G1/2 vomiting and nausea #s even with anti-emetics. Not sure this drug can go forward with that. . #ASCO13
— BiotechStockResearch (@BiotechStockRsr) June 3, 2013
Dr Omlin concluded his presentation by stating that, “the development of this compound by AstraZeneca as a selective androgen receptor down-regulator in mCRPC has been terminated.”
Sometimes promising preclinical data just doesn’t hold up when it moves into human clinical trials. Another AstraZeneca drug with preclinical promise has gone to what Sally Church, PhD (@MaverickNY) refers to as “dog drug heaven.”
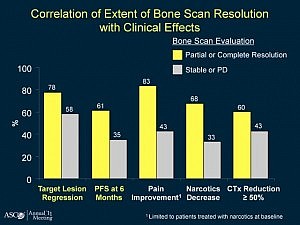
 There is “No data,” Sartor told the ASCO 2012 audience. As a result he recommended the use of less toxic therapies first and that patients be involved in the decision making. Not quite the guidance the audience perhaps hoped for.
There is “No data,” Sartor told the ASCO 2012 audience. As a result he recommended the use of less toxic therapies first and that patients be involved in the decision making. Not quite the guidance the audience perhaps hoped for.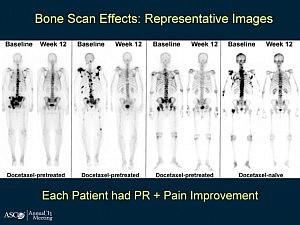


 There was a lot of interest in yesterday’s advanced prostate cancer poster session at EAU 2012.
There was a lot of interest in yesterday’s advanced prostate cancer poster session at EAU 2012. Orteronel (TAK-700) is a selective, non-steroidal inhibitor of 17, 20 lyase, a key enzyme involved in the production of androgens such as testosterone. This is a similar mode of action to
Orteronel (TAK-700) is a selective, non-steroidal inhibitor of 17, 20 lyase, a key enzyme involved in the production of androgens such as testosterone. This is a similar mode of action to 
 After the recent JP Morgan Healthcare conference, San Francisco remains the destination of choice for forthcoming medical meetings.
After the recent JP Morgan Healthcare conference, San Francisco remains the destination of choice for forthcoming medical meetings.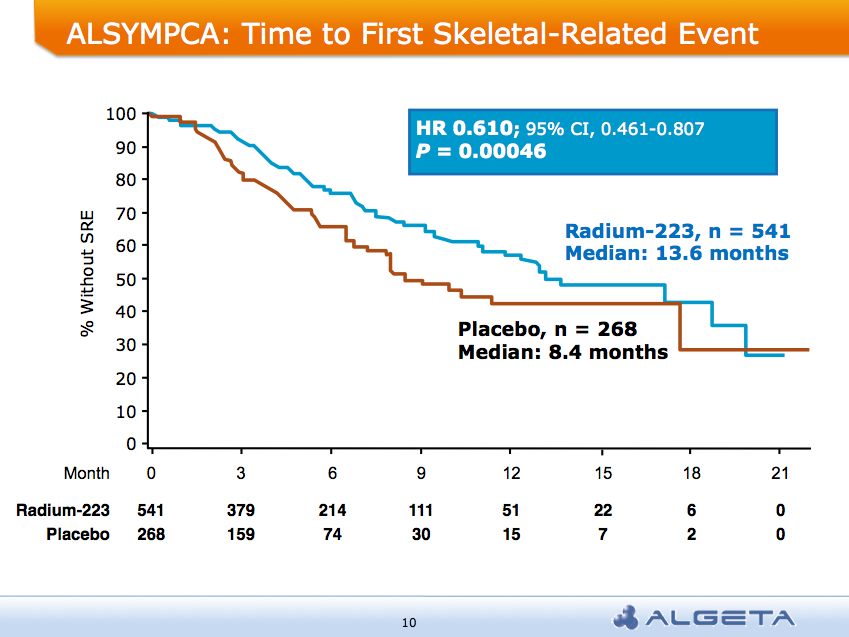 AND a median overall survival of 14 months compared to 11.2 months for placebo group:
AND a median overall survival of 14 months compared to 11.2 months for placebo group: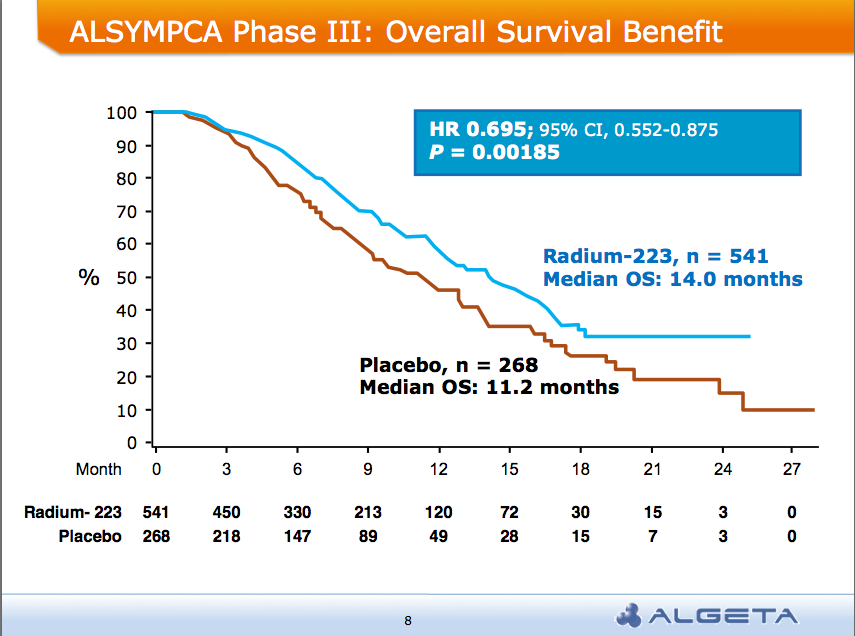 Alpharadin is on the fast track to FDA approval this year
Alpharadin is on the fast track to FDA approval this year This morning the 8am session at the
This morning the 8am session at the  The highlight, in my opinion, was Oliver Sartor’s excellent presentation on radium-223 chloride (Alpharadin) in which he cogently outlined its mechanism of action. He explained that radium-223:
The highlight, in my opinion, was Oliver Sartor’s excellent presentation on radium-223 chloride (Alpharadin) in which he cogently outlined its mechanism of action. He explained that radium-223: