Alpharadin Expanded Access Program is Recruiting #EAU12

Picture with permission of Bayer
No new data on radium-223 (Alpharadin) was presented at the European Association of Urology 2012 Congress in Paris today.
Dr Chris Parker presented a poster with similar data to his oral presentation on Alpharadin at the recent ASCO GU meeting. The phase III ALSYMPCA trial results were first presented at ECCO/ESMO in Stockholm last year.
However, one of things I did learn at EAU12 was that Bayer have opened an Expanded Access Program for Alpharadin, that allows eligble advanced prostate cancer patients access to this radiopharmaceutical pending regulatory approval.
I was told by a Bayer representative that a license is required but that they are now approving sites so that they can administer Alpharadin in the United States pending regulatory approval.
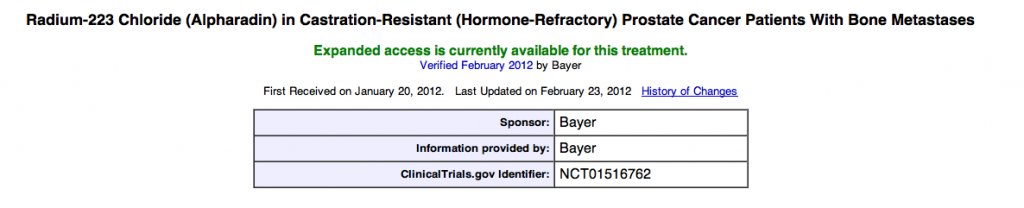
Further information is available about the trial (NCT01516762), and inclusion/exclusion criteria are available on the clinicaltrials.gov website. This is good news for advanced prostate cancer patients.
2 Responses to “Alpharadin Expanded Access Program is Recruiting #EAU12”
NOT QUITE recruiting yet – Hopefully soon. Sites will probably pop up gradually, and it’s very possible that Tulane will be one of the first sites. Just FYI…
The Expanded Access trial for Alpharadin can be found on http://www.CLINICALTRIALS.gov.
The name of the trial is “Radium-223 Chloride (Alpharadin) in Castration-Resistant (Hormone-Refractory) Prostate Cancer Patients With Bone Metastases”. Keep cheking the trial link for updates – every couple of days.
Remember, too, that all prostate cancer patients will not be eligible. There are several criteria, starting with at least 2 bone metastases from prostate cancer.
Jan,
Many thanks for your comment. A representative of Bayer US told me they were approving appropriately licensed centers and that the EAP trial was open for recruitment, so I can only go on what I’m told. The clinicaltrials.gov site isn’t always completely up to date. If no patients have yet been recruited then it sounds like this will change very soon.
I would expect the ALSYMPCA trialists to be part of the EAP program, they are already licensed and have experience of Alpharadin. However, I would expect Bayer is keen to broaden the experience with Alpharadin prior to launch, and my impression is they were open to new sites.
As for the eligibility criteria, you are right the trial is not appropriate for all prostate cancer patients. The clinicaltrials.gov site has detailed information that those interested can discuss with their physicians.
Thanks again for making a comment.
Pieter
Comments are closed.