ESMO 2012: TH-302 Pancreatic Cancer Survival Data Fails to Impress
 At the 2012 Congress of the European Society for Medical Oncology (ESMO 2012) in Vienna, Mitesh J. Borad MD, Assistant Professor of Medicine at the Mayo Clinic in Scottsdale, AZ presented the results of the TH-CR-404 phase 2 clinical trial that compared the efficacy and safety of TH-302 (Threshold Pharmaceuticals) plus gemicitabine versus gemcitabine alone in patients with untreated advanced pancreatic cancer.
At the 2012 Congress of the European Society for Medical Oncology (ESMO 2012) in Vienna, Mitesh J. Borad MD, Assistant Professor of Medicine at the Mayo Clinic in Scottsdale, AZ presented the results of the TH-CR-404 phase 2 clinical trial that compared the efficacy and safety of TH-302 (Threshold Pharmaceuticals) plus gemicitabine versus gemcitabine alone in patients with untreated advanced pancreatic cancer.
TH-302 is an experimental cancer drug in development that kills cells i.e. is cytotoxic under conditions of low oxygen (hypoxia) found in the microenvironment of cancer tumors. Sally Church, PhD on Pharma Strategy Blog has written about the mechanism of action for TH-302 and the results presented earlier this year at the 2012 annual meeting of the American Association for Cancer Research (AACR).
Subscribers to Premium Content can login to read about the ESMO 2012 results and why the results fail to impress.
This content is restricted to subscribers
In the phase IIb study presented at AACR 2012, TH-302 at a dose of 240mg/m² showed a progression free survival benefit of 2 months over gemcitabine. I encourage you to read her article.
ESMO 2012 Results
At ESMO 2012 in the Vienna, the TH-CR-404 data presented (abstract 6660 – full presentation available on the Threshold Pharmaceutials site) showed a benefit of 2.4 months in median progression free survival (PFS) for those patients receiving TH-302 at a dose of 340mg/m2 + gemcitabine compared to gemcitabine alone. The data is summarized in the following table:
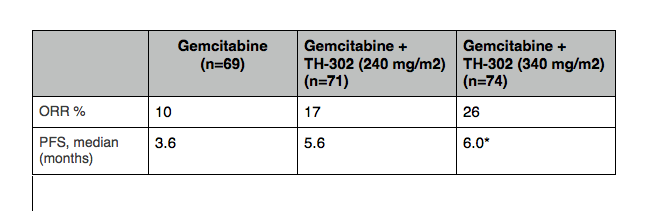
Table adapted from Ducreux, Poster Discussant ESMO 2012. * indicates statistically significant.
The PFS was only significant with the 340mg/m² dose and overall survival (OS) was not significant for either dose. However it should be noted that OS was not a primary endpoint for the study, nor was it powered to detect OS.
Hematological Toxicities may be difficult to handle
The 2.4 month (median) increase in PFS seen with TH-302 + gemcitabine does come with increased toxicities compared to gemcitabine alone. These include an increase in Grade 3 or 4 thrombocytopenia (63%), and increase in Grade 3 or 4 neutropenia (60%). As a UK oncologist sitting next to me said after the presentation, “it is interesting, but quite toxic.” The following table shows some of the key toxicities:
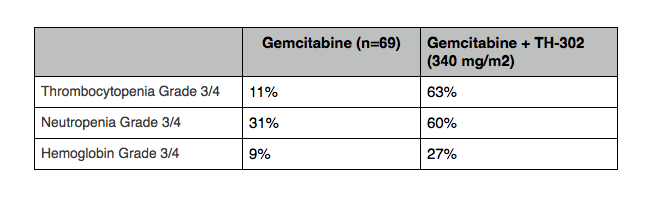
Table adapted from Ducreux, Poster Discussant ESMO 2012.
Threshold plan to start a Phase 3 Trial
Dr Borad told the ESMO 2012 audience that Theshold planned to initiate a randomized phase III trial of TH-302 in advanced pancreatic cancer with the 340mg/m² dose.
Presentation Discussion at ESMO 2012
Michel Ducreux, Head of the Institut Gustave Roussy, Villejuif, France and Professor of Medicine at the University of Paris Sud was the discussant and put the data in context for the audience:
Some of the key points he made were:
- A drug that targets hypoxia may be interesting in pancreatic cancer.
- It was a well done, randomized phase 2 study
- Concern about high level of hematological toxicity.
Professor Ducreux told the audience the hematological toxicity of TH-302 + gemcitabine would be difficult to handle in the many small hospitals in France if it becomes a standard of care. For these reasons, it also means such a combination may be disliked by community oncologists in the United States. Similarly, FOLFIRINOX has shown efficacy versus gemcitabine, but more widespread uptake in first line has been limited by the severe toxicities (including chronic diarrhea) induced by the combination and is therefore not well liked by many oncologists.
“The results are not too bad” said Ducreux. However, he went on to to say “we have to remain suspicious” because positive phase 2 results can be followed by a totally negative randomized phase III trial. He gave axitinib as an example.
“The treatment of pancreatic cancer remains very difficult and I am happy to see new results, but again we have to stay on evidence based medicine, and it is good a phase 3 is planned to evaluate this new compound,” said Ducreux.
Why the results fail to impress
The ESMO 2012 data for TH-302 does, however, have to be considered in the light of the fact that there are other on-going trials that could change the standard of care.
One of trials highlighted by Ducreux in his discussion was Celgene’s nab-paclitaxel (Abraxane) phase III trial that I predict will have positive data in the near future. Here’s a link to my Storify from ESMO 2012 on Abraxane that discusses this.
Professor Ducruex told the ESMO 2012 audience that we will have the results for nab-paclitaxel probably for the next ASCO GI.
If nab-paclitaxel were to increase overall survival to 10-12 months, this would become the new standard of care that TH-302 would then have to beat. Based on the TH-302 data presented at ESMO 2012, even if you ignore the hematological toxicity, this is hard to imagine from the phase II study, which is why the data failed to impress me.
However, until we have the TH-302 phase III data we will not know for sure. In the meantime I am looking forward to the possibility of the Abraxane pancreatic data at ASCO GI and a major breakthrough in the treatment pancreatic cancer. The prospect of this happening will offer hope to all those patients with pancreatic cancer, in a disease that for so long has been a graveyard of drug development.
One Response to “ESMO 2012: TH-302 Pancreatic Cancer Survival Data Fails to Impress”
[…] 1) It will provide a new chemotherapy doublet for future trials to be compared to. This will raise the bar in pancreatic cancer for new entrants including Threshold’s agent, TH–302, that is also attracting a lot of attention here at ESMO. […]
Comments are closed.