 After the recent JP Morgan Healthcare conference, San Francisco remains the destination of choice for forthcoming medical meetings.
After the recent JP Morgan Healthcare conference, San Francisco remains the destination of choice for forthcoming medical meetings.
Yesterday, saw the start of the 2012 ASCO Gastrointestinal Cancers Symposium (ASCO GI) at Moscone West from Jan 19-21.
In a few weeks time, the 2012 ASCO Genitourinary Cancers Symposium (ASCO GU) will be held at the San Franciso Marriott Marquis from Feb 2-4.
If you are based in San Francisco, you are at the heart of the action. It’s less optimal if you are East Coast based, unless you need the frequent flyer miles and have a good travel budget!
According to the ASCO GU preliminary program there are eight oral abstracts on prostate cancer that will be presented at the meeting on Thursday, February 2. Here’s my preview of a few that caught my attention:
ASCO GU Abstract #1:
MDV3100 Phase 3 AFFIRM trial results
The first presentation of the MDV3100 AFFIRM phase 3 trial results are a late-breaking abstract and my prediction for the highlight of the prostate cancer session at ASCO GU.
So far, all that is known from the November 3, 2011 press release from Medivation/Astellas is that MDV3100 produced a 4.8 month advantage in median overall survival compared to placebo in men with advanced prostate cancer.
This met the primary endpoint of the phase 3 AFFIRM trial, and the study was stopped early as a result. As the press release notes, MDV3100 provided a 37% reduction in risk of death compared to placebo (hazard ratio = 0.631).
Howard Scher (MSKCC) will present the AFFIRM trial results at ASCO GU, and a closer look at the MDV3100 data is eagerly awaited.
ASCO GU Abstract #6:
Effect of denosumab on prolonging bone-metastasis-free survival (BMFS) in men with nonmetastatic castrate-resistant prostate cancer (CRPC) presenting with aggressive PSA kinetics.
Amgen are seeking a new indication for denosumab (Xgeva) in prostate cancer on the grounds that it prolongs bone metastasis-free survival in men with non-metastatic CRPC. The supplemental Biologics Application (sBLA) for denosumab will be discussed at the Oncologic Drugs Advisory Committee (ODAC) meeting on February 8, 2012.
The results from the phase 3, 147 trial were published in The Lancet last November and showed that use of denosumab delayed time to first bone metastasis by 3.7 months and improved bone-metastasis free survival.
Sally Church on Pharma Strategy Blog wrote about the denosumab 147 data presented at the annual meeting of the American Urological Association (AUA 2011) last year.
However, the challenge that Amgen faces is that they have yet to show that use of denosumab in men with prostate cancer results in an improvement in overall survival. While it may delay the spread of prostate cancer to the bone, the gold standard for all the prostate cancer drugs approved to date has been overall survival.
The 147 trial showed that overall survival was similar between those taking placebo and those receiving denosumab (HR 1.01; 95 percent CI: 0.85, 1.20; p=0.91). Hypernatremia and osteonecrosis of the jaw were also reported with a higher frequency in the denosumab group
It is possible that there may be updated data at ASCO GU, but most likely it will be a review of The Lancet data with some subset analysis.
The FDA Center for Drug Evaluation & Research (CDER) plans to provide a free of charge, live webcast of the February 8, 2012 meeting of the Oncologic Drugs Advisory Committee, so I am looking forward to what the committee makes of Amgen’s filing.
ASCO GU Abstract #7:
Vitamin E & the Risk of Prostate Cancer – updated results of the Selenium and Vitamin E Cancer Prevention Trial (SELECT)
Eric Klein will be presenting updated results from the SELECT trial that were previously reported in the October 12, 2011 issue of the Journal of the American Medical Association (JAMA).
The data showed a 17% increase in prostate cancer risk with Vitamin E supplements. Although the program abstract advertises updated data, I’m not expecting the data to differ dramatically from last year’s JAMA paper.
ASCO GU Abstract #8:
Overall survival benefit and safety profile of radium-223 chloride (Alpharadin), a first-in-class alpha-pharmaceutical: Results from a phase III randomized trial (ALSYMPCA) in patients with castration-resistant prostate cancer (CRPC) with bone metastases.
The ALSYMPCA trial data is being presented for the benefit of attendees who did not hear Oliver Sartor’s presentation on radium-223 (Alpharadin) at the NY Chemotherapy Foundation or hear Chris Parker present the trial data at ECCO/ESMO in Stockholm. This makes strong commercial sense, especially as it’s a product that physicians in the United States may know little about.
I blogged extensively about the ALSYMPCA trial results presented last year, and had the privilege to do an interview with Chris Parker from the Royal Marsden Hospital at the 2011 European Multidisciplinary Cancer Congress (ECCO/ESMO/ESTRO) in Stockholm.
I am not expecting new data to be presented at ASCO GU on radium-223, but it will be interesting to see how the audience views a bone targeted radio-pharmaceutical that unlike denosumab, does provide an overall survival benefit.
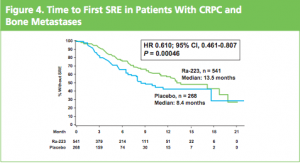
The ALSYMPCA trial showed a significant delay in time to first skeletal-related event (SRE) of 13.6 months vs 8.4 months:
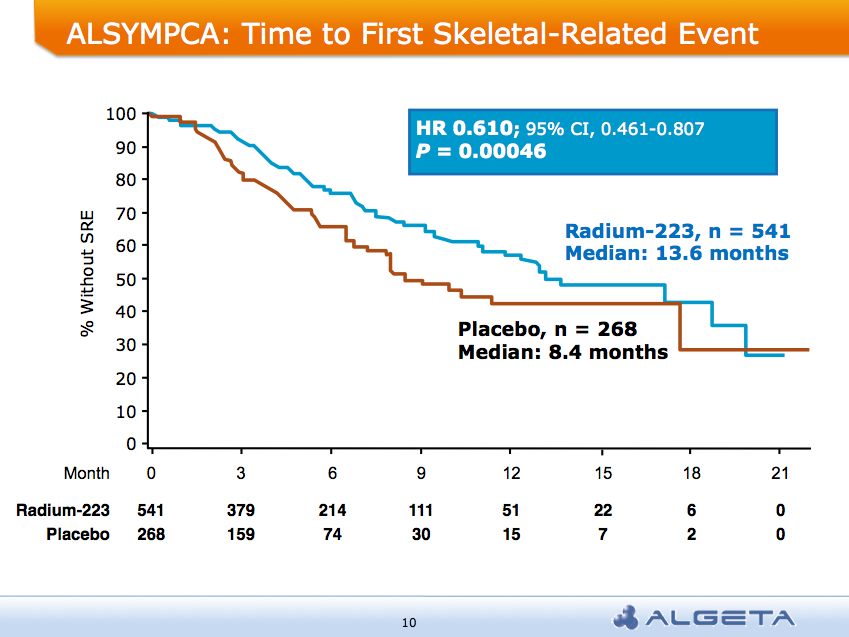 AND a median overall survival of 14 months compared to 11.2 months for placebo group:
AND a median overall survival of 14 months compared to 11.2 months for placebo group:
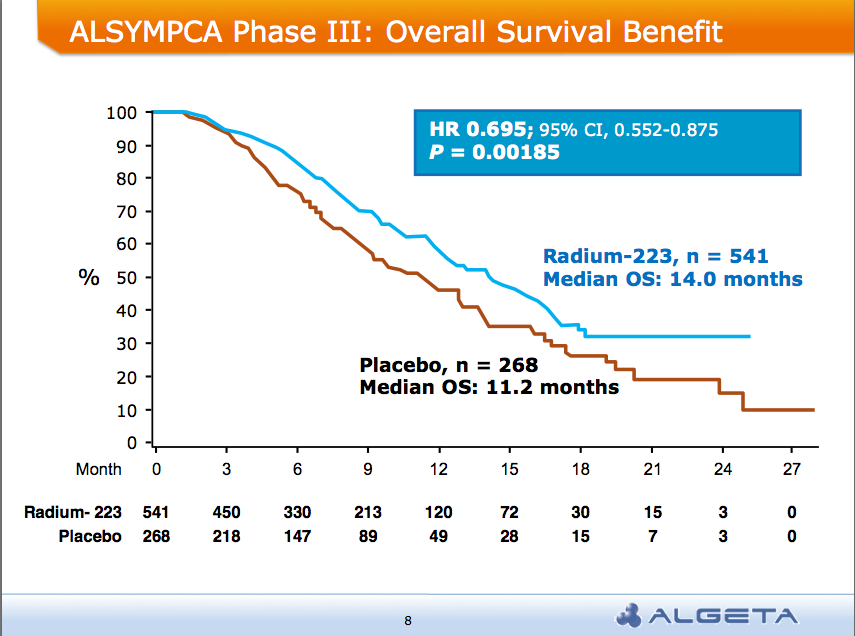 Alpharadin is on the fast track to FDA approval this year.
Alpharadin is on the fast track to FDA approval this year.
My conclusion: If you plan to be at ASCO GU 2012, the prostate cancer data to watch is the first presentation of the MDV3100 AFFIRM trial results.
 There was a lot of interest in yesterday’s advanced prostate cancer poster session at EAU 2012.
There was a lot of interest in yesterday’s advanced prostate cancer poster session at EAU 2012. Orteronel (TAK-700) is a selective, non-steroidal inhibitor of 17, 20 lyase, a key enzyme involved in the production of androgens such as testosterone. This is a similar mode of action to
Orteronel (TAK-700) is a selective, non-steroidal inhibitor of 17, 20 lyase, a key enzyme involved in the production of androgens such as testosterone. This is a similar mode of action to  The
The  After the recent JP Morgan Healthcare conference, San Francisco remains the destination of choice for forthcoming medical meetings.
After the recent JP Morgan Healthcare conference, San Francisco remains the destination of choice for forthcoming medical meetings. AND a median overall survival of 14 months compared to 11.2 months for placebo group:
AND a median overall survival of 14 months compared to 11.2 months for placebo group: Alpharadin is on the fast track to FDA approval this year
Alpharadin is on the fast track to FDA approval this year The recent AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics international conference in San Francisco was an informative meeting.
The recent AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics international conference in San Francisco was an informative meeting. This morning the 8am session at the
This morning the 8am session at the  The highlight, in my opinion, was Oliver Sartor’s excellent presentation on radium-223 chloride (Alpharadin) in which he cogently outlined its mechanism of action. He explained that radium-223:
The highlight, in my opinion, was Oliver Sartor’s excellent presentation on radium-223 chloride (Alpharadin) in which he cogently outlined its mechanism of action. He explained that radium-223: Prostate cancer is the second leading cause of cancer death in men, so it was good news this morning when Medivation & Astellas issued a
Prostate cancer is the second leading cause of cancer death in men, so it was good news this morning when Medivation & Astellas issued a