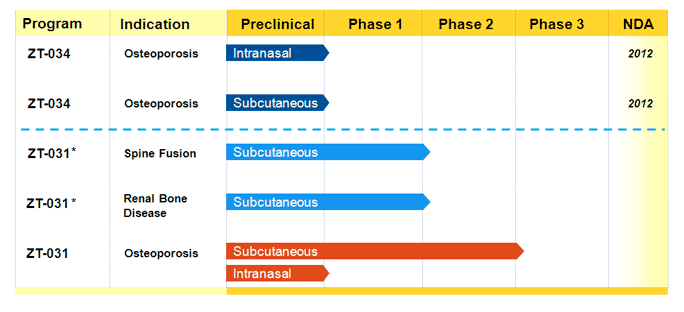
Emerging drugs in development for Osteoporosis
The February 2011 issue of Nature Reviews Drug Discovery has an interesting review by Kawai, Mödder and colleagues on “Emerging therapeutic opportunities for skeletal restoration.”
Some of the new products they discuss include:
- Parathyroid Hormone-Related protein (PTHRP)
- Cathepsin K Inhibitors: odanacatib
- Wnt-ß-catenin pathway targets: sclerostin, DKK1 antagonists, lithium.
The market opportunity for osteoporosis remains significant, affecting 44 million people in the United States over the age of 50, resulting in healthcare costs in excess of $15 billion a year; numbers that are set to increase with the ageing population of baby boomers. The low bone mineral density (BMD) associated with osteoporosis results in increased risk of hip fracture, from which the mortality rate is 20-30% in the first year.
The current competitive landscape for osteoporosis includes antiresorptive agents such as the bisphosponates (alendronate, risedronate, ibandronate, zoledronic acid) that inhibit bone resorption. These compounds reduce fracture-risk by 20-30%, but long-term safety issues remain a concern. High doses of zoledronic acid (Zometa) has been linked to osteonecrosis of the jaw (see previous blog post).
Amgen’s new monoclonal antibody, denosumab, binds to RANK-L, thereby inhibiting its action, with the result that osteoclasts (the cells responsible for bone resorption) cannot form, function or survive. The result of this mechanism of action is a reduction in bone loss and bone destruction.
Like zoledronic acid, denosumab also has a risk of osteonecrosis of the jaw developing. However, one additional long-term safety issue for denosumab is the fact it suppresses TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) that is not only produced by osteoblasts (the cells responsible for bone formation), but also by immune cells. This raises the possibility of skin and immune adverse events, which were seen in the clinical trial data.
Kawai & Mödder in their review article conclude that:
“There is still a need for therapies that reduce fracture risk beyond the level achievable with bone-resorbing agents, particularly as virtually all of the currently available drugs do not eliminate the possibility of future fractures.”
However in addition to having a market opportunity and scientific rationale, any biotechnology company looking at osteoporosis as part of their marketing strategy, must face up to the increasing ethical concerns over placebo-controlled clinical trials. This topic was highlighted last year in the New England Journal of Medicine.
In the future there is likely to be increased pressure not to recruit subjects at high-risk of osteoporosis (T score less than -2.5) into placebo-controlled trials, thus increasing the costs, number of patients and time to bring new products to market. In addition, the regulatory barriers to entry are becoming higher, given that regulatory agencies require a reduction in fractures over 3 years to establish the efficacy of a new drug. This ultimately results in the need for large, expensive, and long phase III clinical trials.
In forthcoming posts, I will discuss the opportunities for market entry by new osteoporosis drugs targeting the Wnt- ß-catenin pathway, Cathepsin K inhibitors and Parathyroid hormone-related protein.