 It’s hard to believe that the countdown to the 2011 annual meeting of the American Society of Clinical Oncology (ASCO) is now underway, but yesterday at 6pm, the ASCO abstracts were released.
It’s hard to believe that the countdown to the 2011 annual meeting of the American Society of Clinical Oncology (ASCO) is now underway, but yesterday at 6pm, the ASCO abstracts were released.
Oliver Sartor at the recent annual meeting of the American Urological Association (AUA) highlighted the prostate cancer potential of cabozantinib (XL184), an oral inhibitor of MET and VEGF kinases, so it was interesting to see that new data will be presented at ASCO.
What makes cabozantinib interesting?
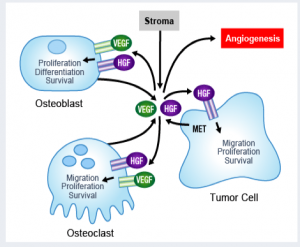
The preliminary data shows that it not only has an anti-tumor effect, but also has an effect on bone metabolism.
The data presented at EORTC last year and at ASCO GU this year confirms what was seen in animal models, in that it had both an anti-metastatic effect on soft tissue and blockade of bone lesions. Such dual action on both bone mets and the tumor microenvironment makes it an exciting new compound in prostate cancer.
By all accounts, the novel effect of cabozantinib on bone mets is unexpected.
At the forthcoming ASCO meeting, abstract 3010, whose lead author is Dr Michael Gordon of Pinnacle Oncology Hematology in Scottsdale, AZ will present data on:
“Activity of cabozantinib (XL184) in soft tissue and bone: Results of a phase II randomized discontinuation trial (RDT) in patients (pts) with advanced solid tumors.”
According to Dr Gordon in the ASCO press teleconference yesterday, the phase II data at ASCO for cabozantinib in prostate cancer will show:
Complete or partial bone scan resolution in majority of patients (86%), often accompanied by pain relief
Unprecedented bone scan improvement
On the basis of these promising results, according to Dr Gordon, “Exelixis plans to initiate the first pivotal trial in prostate cancer by the end of 2011.”
It will be interesting to see whether cabozantinib can impact overall survival (OS) in advanced prostate cancer, something that denosumab (Xgeva®) failed to show in the 147 trial that was just presented at AUA.
There are several abstracts on cabozantinib at the ASCO 2011 annual meeting. Another one that caught my attention was abstract 4516, whose lead author is Maha Hussein of the University of Michigan.
Dr Hussein will present data on cabozantinib in metastatic castrate resistant prostate cancer (mCRPC). The abstract’s conclusion is that:
Cabo showed clinical activity regardless of prior D in mCPRC pts, particularly in pts with bone disease, as reflected by high rates of b-scan resolution and pain relief, in addition to improvements in Hb and tumor regression.
I’ll be at ASCO in a few weeks time, so look forward to hearing more detail on the cabozantinib results. The data is still very preliminary, but cabozantinib (XL184) is certainly a drug to watch, and may be an exciting new prostate cancer drug in the future.
 After exploring the science behind chemotherapy improving T cell trafficking into the tumour yesterday – which is one of the key rate limiting issues that need to be addressed with immunotherapies such as checkpoint blockade – some obvious follow-up questions comes to mind:
After exploring the science behind chemotherapy improving T cell trafficking into the tumour yesterday – which is one of the key rate limiting issues that need to be addressed with immunotherapies such as checkpoint blockade – some obvious follow-up questions comes to mind: It’s Day 7 of our 12 day
It’s Day 7 of our 12 day  After Monday’s look at new developments in the lower GI tract, we now turn our attention today to the upper GI tract with a focus on oesophageal, gastric (stomach), and gastro-esophageal junction (GEJ) cancers.
After Monday’s look at new developments in the lower GI tract, we now turn our attention today to the upper GI tract with a focus on oesophageal, gastric (stomach), and gastro-esophageal junction (GEJ) cancers.
 Recently, new data has begun to emerge that may help inform or enable us to switch to new approaches. While the urologists are eagerly watching the live surgery on the EAU cam, we highlight research data presented at the European Association of Urology (EAU) in Madrid and take a look at how the underlying biology of RCC can elevate our knowledge about where the potential future strategies and blueprint might lie, if we want to facilitate exciting new developments in this field.
Recently, new data has begun to emerge that may help inform or enable us to switch to new approaches. While the urologists are eagerly watching the live surgery on the EAU cam, we highlight research data presented at the European Association of Urology (EAU) in Madrid and take a look at how the underlying biology of RCC can elevate our knowledge about where the potential future strategies and blueprint might lie, if we want to facilitate exciting new developments in this field.


![Reblog this post [with Zemanta]](http://img.zemanta.com/reblog_e.png?x-id=380797cc-c9e2-4168-bf08-f45fb53fbbb6)