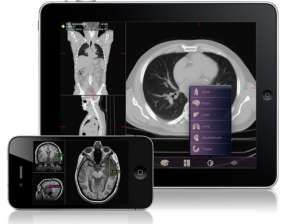
MIM Software receives FDA approval for iPhone and iPad Imaging App
Following on from my blog post last week that discussed the use of iPads and other tablet computers in clinical trials, MIM Software have just received FDA 510(k) clearance to market their iPhone and iPad medical imaging app in the United States. This is the first such approval by the FDA, and the app will be sold in Apple’s itunes store.
This new mobile radiology application will allow physicians to review medical images on their iPhone and iPad. The FDA in their press release indicate that it is not intended to replace full work stations, but to provide the ability to view images and make diagnoses when a workstation is not readily available.
The FDA reviewed luminance, image resolution quality, and results from demonstration studies with radiologists that showed that images could be safely interpreted for diagnostic purposes under appropriate lighting conditions.
What is more, using software from MIM, the images can be further analyzed and distance measurements made.
The ability to have wireless access to medical images will be particularly useful to physicians working remotely, in emergency situations and in clinical trial networks where the central imaging review facility may not be local.
As the screen resolution of iPad’s and other tablet computers increases, perhaps we will see advanced visualization software available on the iPad? It is certainly an area where innovation is taking place, and one that I think will impact clinical research in the biotechnology industry before too long.