How we may be able to rebuild The Six Million Dollar Man
I grew up watching Lee Majors in the 1970’s TV show “The Six Million Dollar Man”, about an injured former astronaut whose bionic implants allowed him to do superhuman feats.
That was fiction, but it is becoming closer to reality as a result of new research into how artificial limbs can integrate with human tissues. This work on neural-electrical interfaces may ultimately allow someone to control a prosthesis as you would a normal limb, i.e. by nerve impulses that travel from the brain.
The promise of “functional integration” between the human nervous system and an electro-mechanical device is the greater control this would give amputees and potential for sensory feedback.
The laboratory of D. Kacy Cullen, Ph.D, assistant professor in the Department of Neurosurgery, Center for Brain Injury & Repair at the University of Pennsylvania, Perelman School of Medicine is actively working in this area. I had the pleasure to hear Dr Cullen talk about his research at Health Journalism 2011 earlier this year. I previously wrote on this blog about his research on nanomaterials that change color with blast impact.
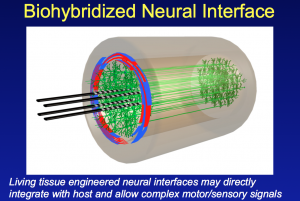
In his presentation to the Association of Health Care Journalists he described some of the neural tissue engineering work in his laboratory. This research has shown the ability to integrate axons in the peripheral nervous system (PNS) with an array of electrodes embedded in a living collagen matrix.
In essence this is the development of a nerve tissue/electrical interface that potentially allows the integration of man and machine.
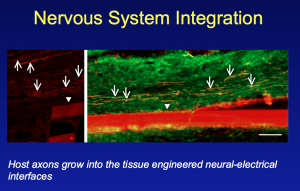
Key to success has been the development of a living, 3-D scaffold where this integration between nerve and electrodes can take place. Nerve axons in the peripheral nervous system require a living target for innervation. This has involved designing and engineering a 3-D living cellular matrix/scaffold that will work within a living body (to date the research has been on animal models).
Not only is Cullen and his laboratory looking at machine/nerve interfaces, but they have also developed techniques that may allow nerves to be repaired. They have shown that nerves can be elongated or stretched using novel tissue engineering techniques.
The resulting axonal constructs via stretch-growth have been transplanted into rats and used to bridge an excised segment of sciatic nerve. What was subsequently seen was an interwining plexus of host and graft axons, suggesting axonal regeneration across the lesion.
While still early stage, and not yet tested in humans, this research has tremendous potential for those paralysed due to traumatic nerve damage in the future.
Moving forward, the Penn researchers aim to develop an application for CNS tissue repair that can be delivered to the brain or spinal cord via stereotactic microinjection. This is minimally invasive surgery that allows delivery of cells to a precise location using 3D co-ordinates.
According to research published in Critical Reviews™ in Biomedical Engineering they plan to use micro-engineered hydrogel conduits, several centimeters long and the width of three hairs. These hydrogels will contain living axonal tracts that will then hopefully reconnect damaged nerves, and provide a platform or path for regeneration.
Many challenges still remain to be worked out for the tissue engineered neural constructs such as issues relating to inflammation and immune tolerance.
The research also needs to move from animals to humans, and be shown to be safe. The long-term functional outcome is also unknown. It is far too early to think of this as a treatment option.
However, advances in neural tissue engineering, neuroregeneration and neuro-prosthetics do offer a lot of promise and hope to the many patients who suffer from spinal cord injuries or loss of a limb.
In a short blog post, I have not been able to do full justice to the innovative research or fully describe the techniques and methodology. More information on the fascinating work being done at Penn, along with details of the associated scientific publications, can be found on the web page of the Cullen Laboratory: Neural Engineering in Neurotrauma.
![]() D. Kacy Cullen, John A. Wolf, Douglas H. Smith, & Bryan J. Pfister (2011). Neural Tissue Engineering for Neuroregeneration and Biohybridized Interface Microsystems In vivo (Part 2) Crit Rev Biomed Eng., 39 (3), 243-262
D. Kacy Cullen, John A. Wolf, Douglas H. Smith, & Bryan J. Pfister (2011). Neural Tissue Engineering for Neuroregeneration and Biohybridized Interface Microsystems In vivo (Part 2) Crit Rev Biomed Eng., 39 (3), 243-262