Can we use immunotherapy to treat or delay Alzheimers Disease?

Preserved section of the Berlin Wall
I have a personal interest in Alzheimers Disease, my mother Audrey died from it three years ago back in 2014.
Since then, I’ve watched with fascination and excitement the progress made in using the body’s own immune system against cancer. There’s still a long way to go, but a revolution in treating cancer is underway, as we’ve been documenting on this blog and the Novel Targets Podcast.
In recent years in the United States we’ve also seen grand initiatives targeting cancer such as Vice President Biden’s Moonshot, as well as large philanthropic support e.g. the creation of the Parker Institute for Cancer Immunotherapy.
Sadly, we’ve not seen the same level of interest in targeting dementia or funding research into new treatments for Alzheimers disease.
In the United States, the media doesn’t talk much about Alzheimers (compared to cancer), unlike for example, in the United Kingdom where any promising data is heralded with headlines that frequently deliver “hype over hope.”
Alzheimers is an insidious disease that removes the ability of the person to advocate and care for themselves, instead placing the burden on families and caregivers, often for extended periods of time. Ultimately many people end up in supported living or nursing homes.
As we debate healthcare insurance in the United States, who is going to pay for the cost of dementia care as the population grows older? Caring for dementia is arguably the greatest public health challenge that the western world faces.
Which is why I was excited to talk with a researcher who is thinking outside of the box and leading the way in how we could use our immune system against Alzheimers.
Subscribers can login or you can purchase access to BSB Premium Content.
This content is restricted to subscribers







 At the recent 2017 American Association for Cancer Research (AACR) annual meeting, Dr Whiteside gave two fascinating talks in education symposia. Afterwards, she kindly spoke to BSB about her research.
At the recent 2017 American Association for Cancer Research (AACR) annual meeting, Dr Whiteside gave two fascinating talks in education symposia. Afterwards, she kindly spoke to BSB about her research. Long time attendees at the annual meeting of the American Association for Cancer Research (AACR) know that there are usually interesting posters and sessions buried on the last day of the meeting.
Long time attendees at the annual meeting of the American Association for Cancer Research (AACR) know that there are usually interesting posters and sessions buried on the last day of the meeting.
 At AACR17, Dr Chen kindly spoke to BSB about his vision for cancer immunotherapy. Anyone who has seen the film, “Jerry Maguire” starring Tom Cruise will remember the moment when Jerry drafts the memo on “The future of our business.”
At AACR17, Dr Chen kindly spoke to BSB about his vision for cancer immunotherapy. Anyone who has seen the film, “Jerry Maguire” starring Tom Cruise will remember the moment when Jerry drafts the memo on “The future of our business.”

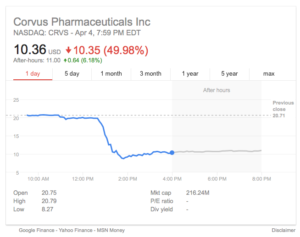 Tuesday at AACR17 was a day when the Corvus Pharmaceuticals stock dropped 50% following presentation of preliminary clinical data for their A2A receptor antagonist CPI-444.
Tuesday at AACR17 was a day when the Corvus Pharmaceuticals stock dropped 50% following presentation of preliminary clinical data for their A2A receptor antagonist CPI-444.