Innovation is seeing round the corners
Innovation involves insight that allows you to see around the corners. That’s the perspective according to Andrew Marks, Professor of Physiology & Cellular Biophysics at Columbia University Medical Center, who recently wrote a Commentary on Innovation in Science Translational Medicine.
Entitled “Repaving the Road to Biomedical Innovation Through Academia”, Professor Marks’ commentary captures the reader’s attention in the first sentence:
“The path to biomedical innovation requires a synthesis of seemingly unrelated observations.”
He goes on to say, “innovation requires joining the pieces to solve the puzzle.”
Innovation according to Marks is difficult to define, something I also noticed at BIO 2011 in the industry panel that I attended.
However, like pornography, “we know it when we see it” to paraphrase Justice Potter. Mark gives examples of innovation in the biological sciences: germ theory of disease by Lister, discovery of antibiotics exemplified by Fleming, Watson & Crick’s work on the structure of DNA.
I don’t disagree that these are examples of paradigm shifting scientific discovery fueled in some cases by serendipity. But are they the best examples of innovation in the biological sciences? Has nothing innovative happened in the past 50 years that is worth mentioning?
In his commentary, Marks goes on to outline the reasons he thinks biomedical research is threatened in the current environment. This includes the standard litany of woes expressed by many academics today:
- increased costs
- insufficient support
- limited industry support
- prolonged postdoctoral training
- limited opportunities for research careers in academic medicine
Interestingly, however, he suggests that part of the fault for this lies with academia.
Academia and the National Institutes of Health (NIH) have failed to evolve with the times, he writes. They “have been guilty of a lack of innovation” in how they support science.
Today’s challenge according to Marks is the need to balance revolutionary research that is innovative with incremental research necessary to further knowledge.
Marks goes on to say that the NIH is not well equipped to judge innovative groundbreaking research. Moreover, “the unwritten rule, often said tongue in cheek, is that when applying for NIH funding one should only propose experiments that one has already done and for which one can show convincing preliminary data.”
The solution he proposes is to change the way federal funding of biomedical research takes place. The NIH should divert to industry the costs of clinical trials and establish distinct funding mechanisms for high-risk research. I am not sure I agree with this, as many clinical trials would not be funded by industry and translational research is not just about basic science, but is from bench to bedside.
The solution proposed by Marks also predisposes that you can properly assess and judge innovative research when you see it. This is not as easy as it seems. As Marks points out:
“NIH likely would not have funded proposals to test the germ-theory, antibiotic-action, or DNA double–helix hypotheses because these projects either would have been deemed too risky (that is, they have a low likelihood of success) or too speculative (lacking in sufficient “preliminary data”) or because the approach would have been criticized as being misguided.”
Instead of looking for new ways to fund basic science, Marks proposes a rework of the way NIH funds research. Cutting the same cake in a different way is unlikely to solve the fundamental problem: there is simply not enough government funding to go around. In the face of the US budget deficit, it is hard to imagine a significant increase in NIH funding to create new funding opportunities.
Would a more innovative approach be to ask academics to rethink how research is funded in their institutions? Focusing on the NIH and Federal Government funding is not the optimal solution in my opinion.
Marks is right in that Academia needs to innovate how science is supported. Incremental change of the way NIH funding takes place may fill in some potholes, but will not repave the road to biomedical innovation.
![]() Marks, A. (2011). Repaving the Road to Biomedical Innovation Through Academia Science Translational Medicine, 3 (89), 89-89 DOI: 10.1126/scitranslmed.3002223
Marks, A. (2011). Repaving the Road to Biomedical Innovation Through Academia Science Translational Medicine, 3 (89), 89-89 DOI: 10.1126/scitranslmed.3002223
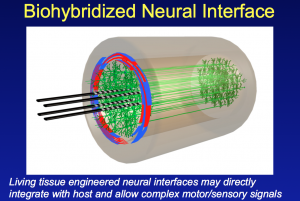
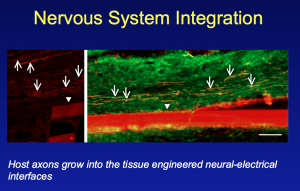

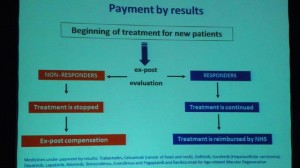
 With this in mind, there’s a series of receptions, parties and events that take place around BIO. Yesterday late afternoon, I attended a reception on the
With this in mind, there’s a series of receptions, parties and events that take place around BIO. Yesterday late afternoon, I attended a reception on the  In the evening the official BIO reception took place at the
In the evening the official BIO reception took place at the  The most enjoyable part of Day 1 of BIO 2011 for me was the unofficial tweetup at the Old Dominion Brewhouse. Who are the people I have been interacting with on Twitter? Some have twitter handles close to their name, others like me are more cryptic. So at a tweetup it’s common to introduce yourself through the language of twitter, “I’m
The most enjoyable part of Day 1 of BIO 2011 for me was the unofficial tweetup at the Old Dominion Brewhouse. Who are the people I have been interacting with on Twitter? Some have twitter handles close to their name, others like me are more cryptic. So at a tweetup it’s common to introduce yourself through the language of twitter, “I’m