BIO 2011: Challenge of innovation in the biotechnology industry
 One of the sessions at BIO 2011 in Washington DC that I hope to make if my travel plans permit, is the Monday afternoon session on “What is the Future for Innovative Medicines in Our Industry’s Pipeline?”
One of the sessions at BIO 2011 in Washington DC that I hope to make if my travel plans permit, is the Monday afternoon session on “What is the Future for Innovative Medicines in Our Industry’s Pipeline?”
The June issue of Nature Reviews “Drug Discovery” attempts to answer this question by looking back at what happened to the R&D projects involving 28,000 compounds investigated since 1990.
Fabio Pammolli and colleagues analyzed the Pharmaceutical Industry Database (PhID) maintained by the IMT (Institutions, Markets, Technologies) in Lucca, Italy.
In their Drug Discovery article entitled “The productivity crisis in pharmaceutical R&D,” they reach a number of conclusions, some of which are:
- Output of new drugs has not matched investment in R&D
- Therapeutic innovation has become more challenging and complex
- Decline in R&D productivity is associated with investments in R&D areas where risk of failure is high
- There is no evidence of any R&D productivity differences between United States and Europe.
The authors analyzed R&D investment decisions by looking at the potential pay-off for an R&D project (probability of market launch multiplied by potential market value) and the expected Probability of Success (POS) in reaching the market based on the average success rate of compounds with the same pathology.
What I found interesting in their paper was the fact that many of the therapeutic areas with the highest percentage of R&D projects had the lowest average POS e.g. cancer drugs (antineoplastic and immunomodulating agents) had the lowest POS (1.8%) and the highest share of total projects (21.77% from 1990 to 1999, increasing to 29.77% from 2000-2007). The 1.8% average probability of success can be contrasted with 4.19% for musculoskeletal system drugs and 6.64% for dermatologicals.
The authors argue that the data shows a shift towards therapeutic markets with a lower POS. What are the reasons for this? Possible explanations include:
- Orphan drug development incentives: legislation that provides incentives to undertake drug development for rare diseases (orphan drugs) has led to a shift towards these targets, which by definition have smaller markets.
- Development of drugs for chronic diseases e.g. Alzheimer’s disease: Collectively these have a POS of 6.88% compared to the acute disease average POS of 8.77%. 85.80% of R&D projects from 2000-2007 were within this category.
- More research targeting lethal diseases such as cancer and infectious disease, which have an average POS of 5.54% compared to non-lethal diseases, average POS of 9.72%.
The authors conclude from this research that:
“R&D investments tend to focus on new therapeutic targets, which are characterized by high uncertainty and difficulty, but lower post-launch competition.”
This article offers some interesting retrospective analysis, but I am concerned that they may have underestimated the market potential for many rare disease areas where market size cannot properly be quantified.
As Novartis showed with imatinib (Gleevec®/Glivec®), it is possible to build a blockbuster out of a very small, rare market (only 4,500 – 5,000 new diagnoses of CML per year in the United States), creating a new market segment and moving the leukemia from a certain death sentence to a chronic disease that can be easily managed with targeted therapy.
The focus of many biotechnology and biopharmaceutical companies on orphan drug development has been shown to be a valid strategy by Genzyme and others. Proving you can bring a product to market and obtain some revenue is likely to stimulate more company investment rather than less.
In the run up to BIO 2011 several companies have highlighted their orphan drug strategy, including Oklahoma City based Selexys Pharmaceuticals who announced news about SelG1 in Sickle Cell Disease and Lamellar Biomedical from Glasgow with LMS-611 for Cystic Fibrosis.
I am looking forward to learning more at BIO on how industry experts view the future for innovation within the sector. Also whether the orphan drug strategy that many biotech companies are now following will pay off given the lower probability of success in rare indications.
All in all, the 2011 BIO international convention is set to be an interesting and informative meeting. Business cards, comfortable shoes and camera/video – I’m ready!
![]() Pammolli, F., Magazzini, L., & Riccaboni, M. (2011). The productivity crisis in pharmaceutical R&D Nature Reviews Drug Discovery, 10 (6), 428-438 DOI: 10.1038/nrd3405
Pammolli, F., Magazzini, L., & Riccaboni, M. (2011). The productivity crisis in pharmaceutical R&D Nature Reviews Drug Discovery, 10 (6), 428-438 DOI: 10.1038/nrd3405


 At the 16th Congress of the
At the 16th Congress of the  As
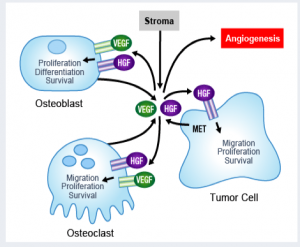
As  The market for prostate cancer therapies is set to expand from $1 billion currently to $5 billion by 2015, according to analysts reported by
The market for prostate cancer therapies is set to expand from $1 billion currently to $5 billion by 2015, according to analysts reported by  As many of you know, I previously wrote up on this blog
As many of you know, I previously wrote up on this blog 
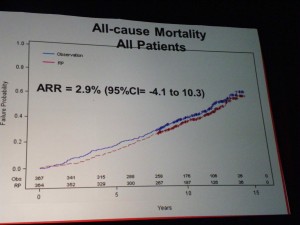 All-Cause Mortality
All-Cause Mortality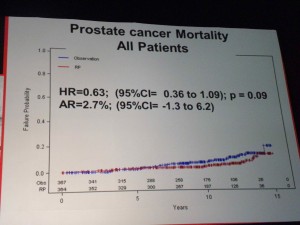 Prostate Cancer Mortality – all patients
Prostate Cancer Mortality – all patients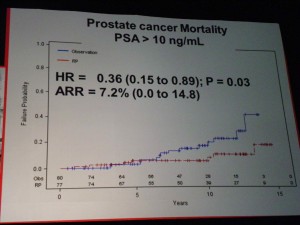 Only in the high-risk groups (PSA>10) was there a significant benefit to RP, in terms of lowering Prostate Cancer Mortality.
Only in the high-risk groups (PSA>10) was there a significant benefit to RP, in terms of lowering Prostate Cancer Mortality.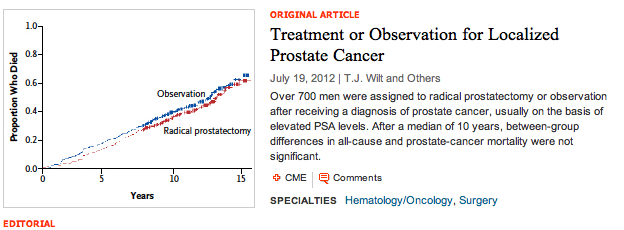
 One of the sessions that I attended at the 2011 annual meeting of the American Urological Association (AUA) focused on research into advanced prostate cancer. A particularly thought provoking presentation was:
One of the sessions that I attended at the 2011 annual meeting of the American Urological Association (AUA) focused on research into advanced prostate cancer. A particularly thought provoking presentation was: Presented by Alex Haese from Hamburg, Germany, this paper was a retrospective analysis of 1,574 patients who had a biochemical recurrence (PSA > 0.2 mg/dl) following RP. Researchers looked at clinical progression and cancer specific survival rates and compared their findings to published United States data.
Presented by Alex Haese from Hamburg, Germany, this paper was a retrospective analysis of 1,574 patients who had a biochemical recurrence (PSA > 0.2 mg/dl) following RP. Researchers looked at clinical progression and cancer specific survival rates and compared their findings to published United States data.