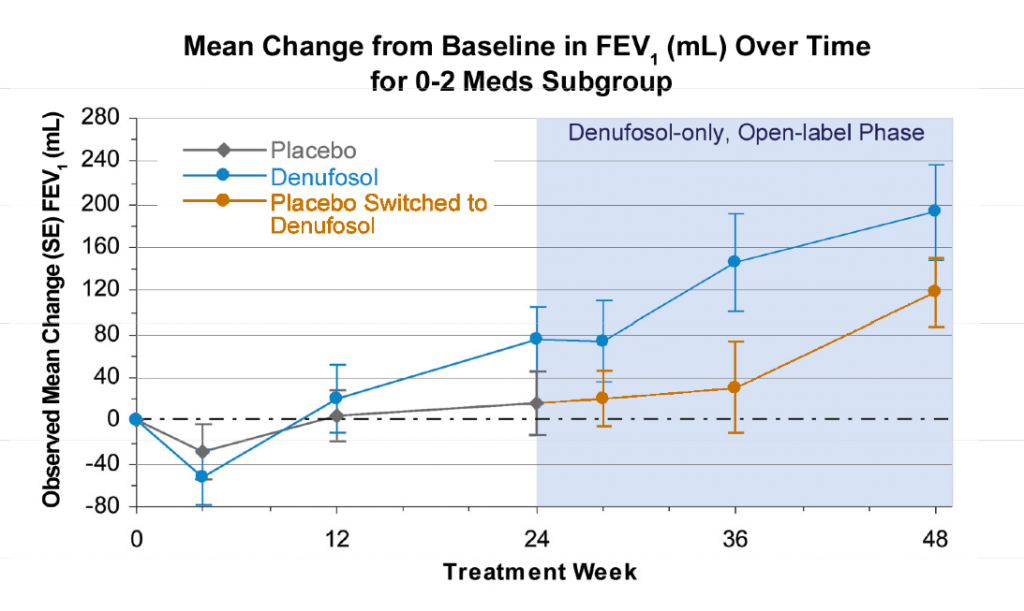
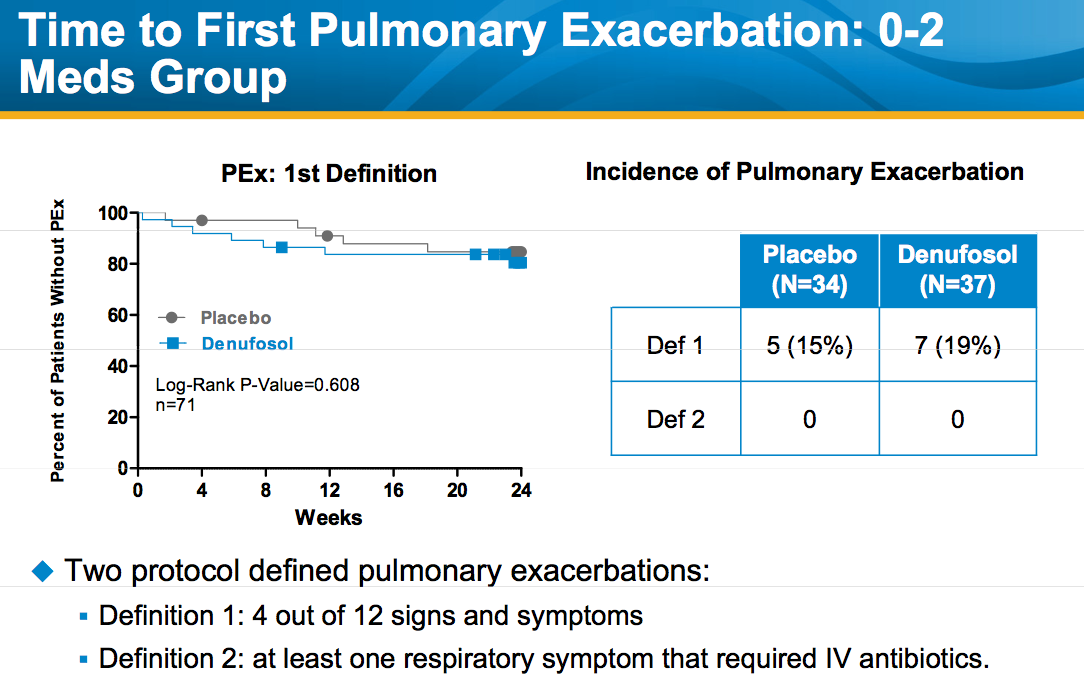
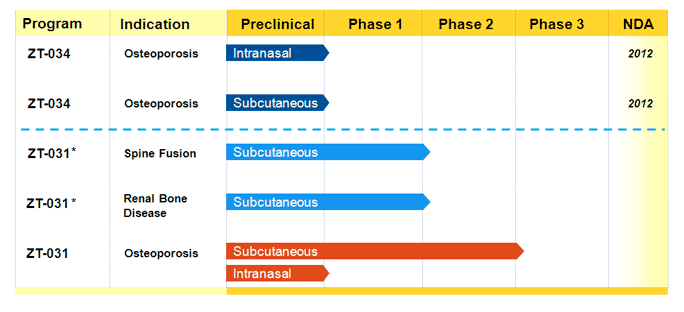
I recently attended the annual meeting of the American Society of Hematology (ASH) in Orlando so thought I would share my general impressions (in no particular order):
I recently attended the annual meeting of the American Society of Hematology (ASH) in Orlando so thought I would share my general impressions (in no particular order):
Convention Center Food was Poor:
The food at the Orange County Convention Center reminded me of an airport – desperate choices, poor quality and overpriced. As for no Starbucks or decent coffee shop, civilization has yet to reach Orlando! The Peabody hotel across the road had coffee shops that offered a $3 single espresso shot, but they ran out of pods on the Monday morning – faced with the overwhelming demand that seems to have not been anticipated! Memo to Starbucks – look into a convention center franchise.
Cramming all the science in one day doesn’t work:
People go to a meeting such as ASH for many reasons – networking, business development, education, investigator meetings, but in the end, it is a scientific meeting. The meeting ran from Saturday to Tuesday, yet nearly all the oral scientific program (biology and therapy) was crammed into one day of simultaneous sessions on the Monday – tough if you wanted to cover a product or pathway that targeted multiple therapeutic areas, many of which ran at the same time.
The Poster hall was like a graveyard:
You can tell I didn’t think this was a great meeting. Every time I went into the aircraft like hangar where the posters were housed, I ended up chilled to the bone. It certainly didn’t encourage spending much time there, and I was disappointed that a surprisingly high number of presenters did not attend the poster presentation receptions, when they were supposed to be available to answer questions. Nobody seemed to check if anybody showed up, to me the whole point of a poster is being able to discuss it.
Why not publish the slides from the oral science presentations?
ASH, unlike ASCO does not make the slides of oral scientific presentations available online, so if you didn’t make notes, or break the rules (and risk being ejected) by taking an illegal picture on your phone or camera, then you missed it. Abstracts are often submitted months in advance before the final data is analyzed, so by not making the slides available after the meeting, science to me is being hampered especially given the overlap of sessions on the Monday. If an abstract is presented and published, the scientific information should be available to be shared. Isn’t that what science is about? ASCO have it right, their virtual meeting program is outstanding.
Hospitality lives on – but only if you are an international doctor:
US doctors attending their annual meeting, are warned not to accept a free cup of coffee at an exhibitor booth if their state or employer prohibits the acceptance of such “gifts”, while foreign physician attendees are wined and dined. You see signs for hospitality centers for European doctors or desks in hotels for company sponsored groups of foreign doctors – hard to believe there’s ethically not something wrong with these double standards.
A lack of quality educational materials:
While the free pen and notepad have long since gone the way of the dinosaur, this year there was noticeably fewer educational material to take away. The debate as to whether doctors should pay for their own CME continues, but I do think many in the industry miss the opportunity by not providing educational material on the science, pathways and mechanism of action of new products.
The “Super Friday” is still alive:
Multiple industry sponsored satellite symposia (AKA “Super Friday” sessions) took place before ASH, with several at the Peabody Hotel. I have to say the food at the one I attended on personalized medicine was excellent, one of my best meals in Orlando. They are not cheap to run: not only do 800-1000 people get fed, but a hotel room with audio-visual equipment has to be hired, a panel of experts are paid to talk about a topic, and many of the attendees come away with a glossy brochure. Could this money be better spent elsewhere? ASH has a large education component to it, and it was interesting to note that what the ASH education program committee chose as important topics to talk about and what the industry chose, were pretty different. That is perhaps not surprising – after all if you choose the topic of the satellite symposia and it’s of relevance to your product, indirectly you are trying to influence prescribing behavior, otherwise why else would you fund it? There is no such thing as a free lunch or dinner.
Multiple Myeloma was a hot topic:
There was a lot of interest in clinical trial results in MM. The use of a maintenance therapy, and attempt to turn this into a chronic disease was a widely discussed topic, however many old drugs such as thalidomide have nasty side effect profiles such as peripheral neuropathy, while newer drugs such as lenalidomide are expensive but appear to only incrementally increase survival. Results from multiple combinations of drugs and induction therapies were presented, I was left with the impression that although there is progress, there is still no major breakthrough in this disease area.
Nobody wants to talk about cost effectiveness:
The 800 lb gorilla in the room for hematology/oncology is the comparative effectiveness of one treatment versus another i.e. it’s cost/benefit, yet nobody wants to talk about it. Take for example the treatment of CML, should you treat with imatinib (Gleevec) which has outstanding long-term survival data over several years thanks to the IRIS trial, or use a second-generation tyrosine kinase inhibitor (TKI) such as dasatinib or nilotinib that is around twice the price, more potent and slightly more effective but obviously we don’t yet know if it improves 5 or 10 year survival yet over imatinib. Nobody wanted to talk about price – physicians currently live in an ivory tower.
If you are interested in information on what the hot scientific news was at ASH, then I encourage you to look at the excellent posts (here & here) published by my colleague on Pharma Strategy Blog.