AACR 2013 DMUC5754A is a novel agent for Ovarian Cancer
The cherry blossoms are finally blooming in Washington DC for the 2013 annual meeting of the American Association for Cancer Research (AACR).
With AACR in DC this year, the following traditional Japanese haiku published on the National Park Service website struck me as appropriate for cancer researchers and survivors to reflect on:
Yo no naka wa, Mikka minu ma ni, Sakura kana
“Life is short, like the three day glory of the cherry blossoms.”
Yesterday at AACR was predominantly an educational day, but several studies were highlighted to the assembled media. One of the late-breaking clinical trials that caught my attention was the preliminary phase 1 data on Genentech’s novel new agent DMUC5754A.

Joyce Liu MD MPH
LB-290 Targeting MUC16 with the Antibody-Drug Conjugate DMUC5754A in patients with platinum-resistant ovarian cancer. This data will be presented by Joyce Liu, MD, MPH from Dana-Farber Cancer Institute in the Clinical Trials Symposium on Tuesday, Apr 9 at 4.00 pm.
Dana-Farber issued a press release yesterday – here’s the link. The picture of Dr Liu is from her Dana-Farber profile.
Ovarian cancer causes more deaths in women than any other cancer of the reproductive organs, with an estimated 20,000 women diagnosed with this cancer each year. The majority of women are treated with traditional platinum based chemotherapies, and most of these relapse and develop drug-resistant disease. This makes the development a new novel agent such as DMUC5754A that will treat platinum-resistant ovarian cancer a major potential breakthrough.
In an AACR media release, Dr Liu commented on how the drug works:
“This drug consists of an antibody and a potent toxin joined by a cleavable linker. The antibody identifies a protein, MUC16, which is highly expressed in ovarian cancers, and targets the toxin to kill the cancer cells.”
Liu went on to note that, “Unlike other cancer treatments, the antibody-drug conjugate releases the toxin with relative selectivity to the MUC16-positive cancer cells. This allows delivery of drugs that would otherwise be too toxic for treatment.”
According to Liu, “If the activity of this drug is confirmed in additional trials, this will represent a novel type of therapy for ovarian cancer, with effectiveness in platinum-resistant ovarian cancer, which is the hardest type of ovarian cancer to treat.”
Genentech are particularly good at sharing early data at AACR, and based on the promising responses in MUC16 IHC 2/3+ patients, this new ADC compound is likely to progress to phase 2 – a compound to watch out for in the future.

 In his ASH lecture, Dr June noted that there are side effects and toxicities associated with CTL019 including tumor lysis syndrome (TLS), and Cytokine Release Syndrome (CRS) was seen in all patients.
In his ASH lecture, Dr June noted that there are side effects and toxicities associated with CTL019 including tumor lysis syndrome (TLS), and Cytokine Release Syndrome (CRS) was seen in all patients.  At the December 2012 annual meeting of the American Society of Hematology (ASH) in Atlanta interim data was available from the phase 1 clinical trial of ABT-199 in Non-Hodgkin Lymphoma (
At the December 2012 annual meeting of the American Society of Hematology (ASH) in Atlanta interim data was available from the phase 1 clinical trial of ABT-199 in Non-Hodgkin Lymphoma ( Imagine that you are born deaf and live in a world of silence – what price would you pay for a new treatment that might restore your hearing?
Imagine that you are born deaf and live in a world of silence – what price would you pay for a new treatment that might restore your hearing?
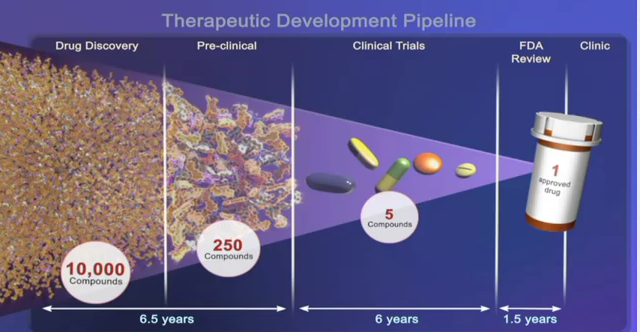
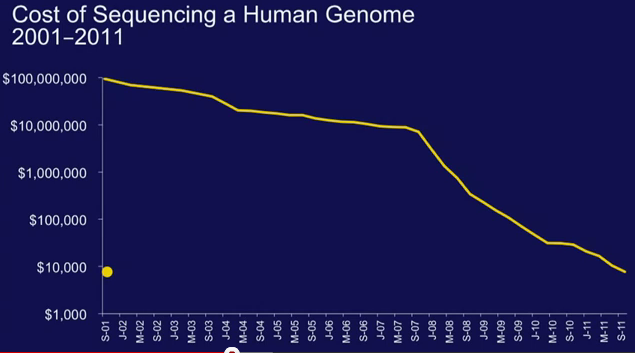
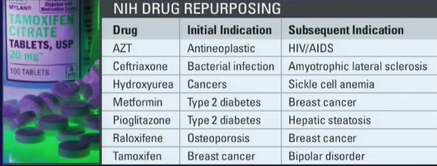

 One enduring image I have from the plenary presentation on “The Genetic Basis for Cancer Therapy” by Bill Sellers, VP/Global Head Oncology at Novartis Institutes for BioMedical Research was the video he showed of the robots that are used for automated cell profiling.
One enduring image I have from the plenary presentation on “The Genetic Basis for Cancer Therapy” by Bill Sellers, VP/Global Head Oncology at Novartis Institutes for BioMedical Research was the video he showed of the robots that are used for automated cell profiling. To do this, Sellers described how Novartis have built a robotic system that e.g. automates cell profiling. In approx 3 months with this system we can profile 600 cell lines for about 1500 compounds, he said.
To do this, Sellers described how Novartis have built a robotic system that e.g. automates cell profiling. In approx 3 months with this system we can profile 600 cell lines for about 1500 compounds, he said.