Vitamin E may improve gemcitabine effectiveness in pancreatic cancer
It’s been a bad week for vitamins, especially with the publication of data from the SELECT trial that showed healthy men taking 400 IU/day of Vitamin E had a 17% increased risk of prostate cancer.
However, there is some evidence in support of tocotrienols (unsaturated form of Vitamin E) having a potential role to play in anti-cancer therapy. One paper that caught my attention was the work by Kazim Husain and colleagues from the Moffitt Cancer Center and Research Institute in Tampa.
Published Online First (October 4, 2011) in the American Association for Cancer Research (AACR) journal, “Molecular Cancer Therapeutics” they showed that δ- tocotrienol may have potential to improve the effectiveness of gemcitabine in pancreatic cancer.
In their laboratory and animal based research, the authors showed that δ-Tocotrienol:
- “augments inhibition of pancreatic cancer cell proliferation by gemcitabine”
- “augments gemcitabine-induced apoptosis in pancreatic cancer cells”
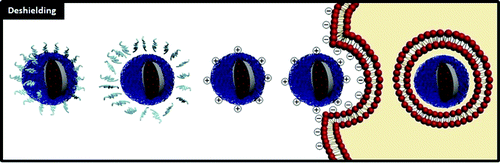
- “down-regulates constitutively activated NF-κB in gemcitabine-treated pancreatic cancer cells”
- “enhances the in vivo therapeutic effects of gemcitabine in a pancreatic tumor model in SCID nude mice”
Pancreatic cancer patients have a poor prognosis with less than <5% of patients surviving 5 years. Current treatment revolves around the chemotherapy gemcitabine, but as the authors note in their Molecular Cancer Therapeutics paper, “tumor resistance is common.”
Various researchers are working on how to improve treatment options for pancreatic cancer. One company I’m watching is AB Science and their phase 3 trial for masitinib. You can read more about this on Pharma Strategy Blog and Sally Church’s excellent interview with CEO, Alain Moussy.
The work on the δ-tocotrienol form of Vitamin E shows that it may have a role to play in cancer treatment, notwithstanding the negative data that was published earlier this week in prostate cancer.
Husain and colleagues from Moffitt showed for the first time that δ-tocotrienol inhibited NF-κB activity and the expression of NF-κB regulated gene products. They note that inflammatory transcription factor NF-κB is involved in tumorigenesis, so inhibition of NF-κB may be how tocotrienols exert their anti-cancer effects.
These preclinical results are promising and show that:
“δ-tocotrienol is the most bioactive tocotrienol against human pancreatic cancer cells and provide the rationale for selecting δ-tocotrienol as the lead tocotrienol compound for further studies of the use of tocotrienols for pancreatic cancer prevention and treatment.”
A phase I clinical trial is ongoing (NCT00985777) evaluating the use of δ-tocotrienol in patients with pancreatic tumors.
While Vitamin E supplementation may yet be of benefit to healthy individuals, it could have benefit in patients with pancreatic cancer, so it will be interesting to see how this develops.
![]() Husain, K., Francois, R., Yamauchi, T., Perez, M., Sebti, S., & Malafa, M. (2011). Vitamin E -Tocotrienol Augments the Anti-tumor Activity of Gemcitabine and Suppresses Constitutive NF- B Activation in Pancreatic Cancer Molecular Cancer Therapeutics DOI: 10.1158/1535-7163.MCT-11-0424
Husain, K., Francois, R., Yamauchi, T., Perez, M., Sebti, S., & Malafa, M. (2011). Vitamin E -Tocotrienol Augments the Anti-tumor Activity of Gemcitabine and Suppresses Constitutive NF- B Activation in Pancreatic Cancer Molecular Cancer Therapeutics DOI: 10.1158/1535-7163.MCT-11-0424



 The market for prostate cancer therapies is set to expand from $1 billion currently to $5 billion by 2015, according to analysts reported by
The market for prostate cancer therapies is set to expand from $1 billion currently to $5 billion by 2015, according to analysts reported by