New cystic fibrosis drug offers hope
Inspire Pharmaceuticals (NASDAQ:ISPH), a North Carolina based biopharmaceutical company that focuses on products for ophthalmic and pulmonary diseases, recently announced positive results from their phase 3 trial (TIGER-1) of denufosol tetrasodium in patients with Cystic Fibrosis (CF).
Cystic Fibrosis is a genetic disorder that can lead to death as a result of pulmonary complications from airway obstruction, bronchial thickening and accumulation of mucous. Lung function tests are widely used in the diagnosis, treatment and management of patients with CF. Measurement of FEV1 (Forced Expiratory Volume in 1 second) is regarded as the best predicator of mortality. As the disease progresses and the lungs become more obstructed, FEV1 decreases.
Inspire Pharma’s denufosol is an ion-channel regulator that helps keep the airways moist and helps mucous removal in CF patients. It increases chloride secretion via calcium-activated chloride channels (CaCCs), inhibits sodium absorption via epithelial sodium channels (ENaCs) and stimulates ciliary beat frequency. Conveniently for patients, it is being developed as an inhaled drug delivered direct to the lungs by nebulizer.
The phase 3 clinical trial data presented by Dr Frank Accurso at the Annual North American Cystic Fibrosis Conference, and in the paper published in the American Journal of Respiratory and Critical Care Medicine (AJRCCM), showed an improvement in lung function after 24 weeks in patients with mild CF who received daily denusofol by means of a nebulizer. The primary efficacy endpoint was a change in FEV1:
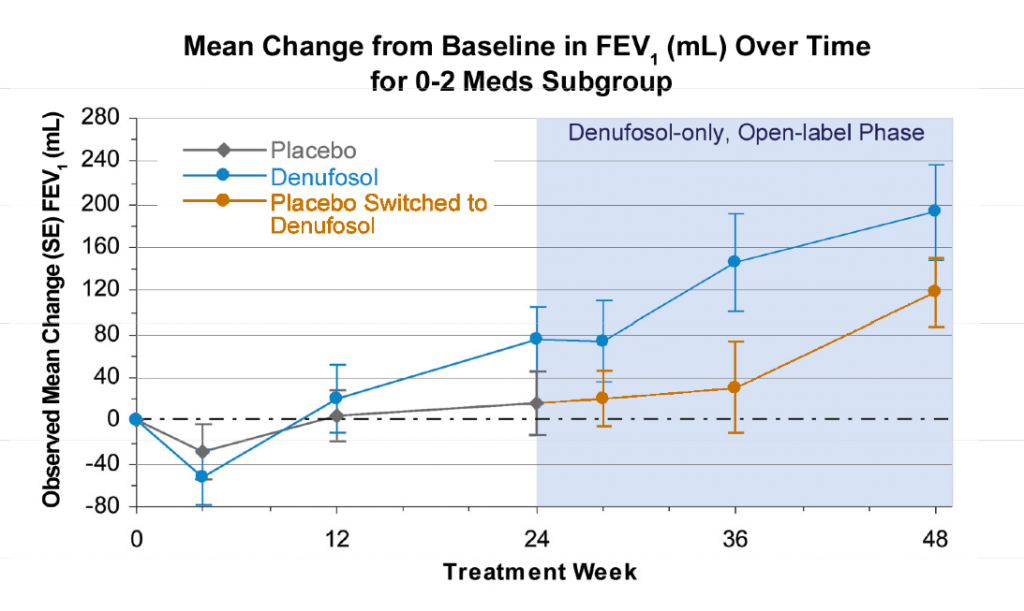
Source: October 21, 2010 presentation by Frank J. Accurso M.D. to North American Cystic Fibrosis Conference. Available at Inspire Pharma.
Dr Accurso and his colleagues reported that the results demonstrated:
“Mean change from baseline to Week 24 endpoint in expiratory volume at 1 second (primary efficacy endpoint) was 0.048 L for denufosol (n=178) and 0.003 L for placebo (n=174; P=0.047).”
Despite the significant improvement in FEV1, there was no significant difference between the denufosol and placebo arms in the time to progression to first pulmonary exacerbation, suggesting that its long-term clinical effectiveness remains uncertain.
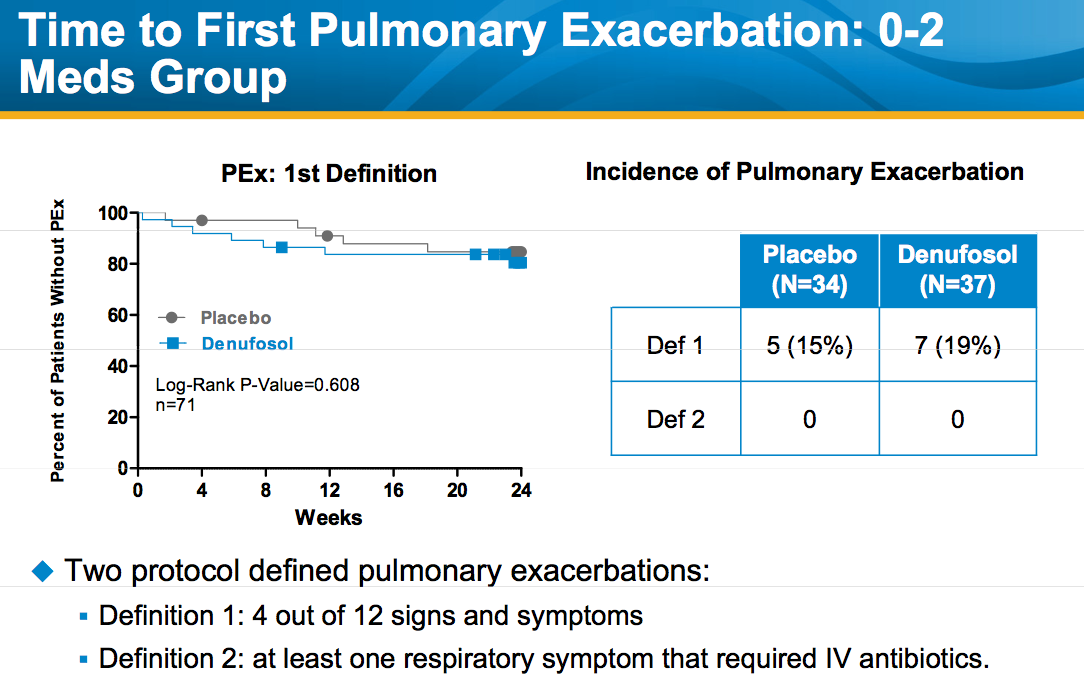
Source: October 21, 2010 presentation by Frank J. Accurso M.D. to North American Cystic Fibrosis Conference. Available at Inspire Pharma.
Notwithstanding, these results do offer hope to patients with mild symptoms of Cystic Fibrosis. Early treatment to maintain lung function may delay the onset of more severe physiological changes and the need for more radical treatment options such as a heart/lung transplant.
Thanks to BBC Health for writing about this topic and giving me the idea for this post.