As 2013 comes to an end, rather than look back as many are doing, I’m looking forward to 2014. January is a busy month for cancer meetings with the ASCO organized gastrointestinal cancers symposium (ASCO GI) and genitourinary cancers symposium (ASCO GU) both taking place in San Francisco a few weeks apart.
In fact, looking at the calendar of forthcomings meetings, 2014 looks to have a West Coast focus, with the annual meeting of the American Association for Cancer Research (AACR) taking place in San Diego in April, and the American Society of Hematology (ASH) annual meeting also heading to San Francisco in December.
Transcontinental airfares are notoriously expensive at the last minute so if flying from the East Coast, do make travel plans early!
The ASCO GU symposium takes place at the San Francisco Marriott Marquis from Jan 20 – February 1, 2014. The abstracts for meeting go online at 5pm Eastern Time on Jan 28.
ASCO in a December 19 press release have already announced what will be highlighted on the January 28 press cast, and what many of the media can be anticipated to write about from the meeting.
Perhaps not surprisingly the Medivation PREVAIL trial data (LBA1) is top of the list; the abstract for this presentation has already been published online as Professor Tombal (@BertrandTOMBAL) kindly highlighted on Twitter.
This preview highlights some of the prostate cancer abstracts and presentations to watch out for at the meeting:
Drugs discussed in this post include: enzalutamide (Xtandi), abiraterone (Zytiga), ODM-201, ARN-509, ipilimumab (Yervoy).
<h3><span style=”color: #ff6600;”><strong>To learn more about our latest insights, subscribers can <a href=”https://biotechstrategyblog.com/login/” target=”_blank” rel=”noopener”>log-in</a> or you can <a href=”https://biotechstrategyblog.com/pricing/” target=”_blank” rel=”noopener”>purchase access</a> to BSB Premium Content. </strong></span></h3>
This content is restricted to subscribers
 At the recent ASCO 2016 Genitourinary Cancers Symposium (ASCO GU) that took place in San Francisco the week before the JP Morgan Healthcare Conference (JPM), one of the noteworthy presentations was on a novel target for men with advanced prostate cancer.
At the recent ASCO 2016 Genitourinary Cancers Symposium (ASCO GU) that took place in San Francisco the week before the JP Morgan Healthcare Conference (JPM), one of the noteworthy presentations was on a novel target for men with advanced prostate cancer. New Orleans – in today’s plenary session at the
New Orleans – in today’s plenary session at the 



 San Francisco – In the ASCO GU prostate cancer session yesterday morning one of the most interesting presentations was by Andrew J Armstrong, Associate Professor of Medicine and Surgery at the Duke Cancer Institute.
San Francisco – In the ASCO GU prostate cancer session yesterday morning one of the most interesting presentations was by Andrew J Armstrong, Associate Professor of Medicine and Surgery at the Duke Cancer Institute. At ASCO GU this week (
At ASCO GU this week (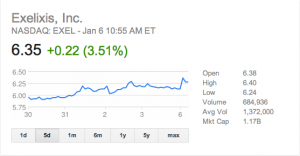 The share price of Exelixis ($EXEL) is starting a run-up (after months in the doldrums) in advance of anticipated results from the COMET-1 phase 3 trial in metastatic castrate resistant prostate cancer (mCRPC) for cabozantinib (Cometriq, formerly XL184).
The share price of Exelixis ($EXEL) is starting a run-up (after months in the doldrums) in advance of anticipated results from the COMET-1 phase 3 trial in metastatic castrate resistant prostate cancer (mCRPC) for cabozantinib (Cometriq, formerly XL184).