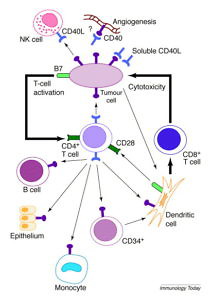
CD40 as a Cancer Immunotherapy Target
There are now several CD40 agonist antibodies in early clinical development from several different companies, including:
- Roche – RO7009789
- Apexigen – APX005M
- Seattle Genetics – SEA-CD40
- Alligator Bioscience – ADC–1013
This post is the last in our cancer immunotherapy coverage from the European Cancer Congress in Vienna. It features excerpts from an interview with Dr Christian Rommel, head of oncology discovery at Roche in Basle, Switzerland in which he talks about the development of their CD40 monoclonal antibody. Readers may recall we wrote about this from SITC 2014 last year: “Targeting CD40 in Cancer Immunotherapy.”
This post is also a new primer on CD40 as we start our coverage of the Society for Immunotherapy of Cancer (SITC) 2015 annual meeting. We’re informed by SITC it’s a sell out conference with 600 more people than last year’s record breaking number. Cancer Immunotherapy is indeed the hottest topic in cancer drug development.
If you have plans to be at National Harbor this week, we hope to see you there!
To learn more insights on this intriguing topic, subscribers can log-in or you can purchase access to BSB Premium Content.
This content is restricted to subscribers

 To maintain the balance between novel targeted agents and immunotherapy, here’s a review of some of the interesting new developments that I came across at AACR, from both the poster halls, as well as some of the thought leaders in this space.
To maintain the balance between novel targeted agents and immunotherapy, here’s a review of some of the interesting new developments that I came across at AACR, from both the poster halls, as well as some of the thought leaders in this space.