 Chicago – at the 2016 annual meeting of the American Society of Clinical Oncology (ASCO), two global prostate cancer experts told me that a phase 3 prostate cancer immunotherapy trial in the pre-chemotherapy setting “read out” as negative last year, but they haven’t seen the data presented at a major medical meeting, published in a journal, and to the best of my knowledge, no press release has ever been issued.
Chicago – at the 2016 annual meeting of the American Society of Clinical Oncology (ASCO), two global prostate cancer experts told me that a phase 3 prostate cancer immunotherapy trial in the pre-chemotherapy setting “read out” as negative last year, but they haven’t seen the data presented at a major medical meeting, published in a journal, and to the best of my knowledge, no press release has ever been issued.
(correction: the trial failure was mentioned in a 2Q 2015 earnings press release on July 23, 2015, but no separate press release was issued in the same way one would have been for positive data. As one senior PR person told me at ASCO, only announcing it in a financial news release, “doesn’t count.”)
I have been following prostate cancer for several years and was surprised by the news (that I and others clearly missed), especially as many in the field had hoped the trial might be positive and that the lack of news was a positive. In event driven trials, a later reaching of a milestone or data read out is usually good news – it means people lived longer!
The company in question is Bristol Myers Squibb, a publicly traded global oncology company. At the ASCO 2016 annual meeting, they are presenting the results of multiple clinical trials that show promising data for checkpoint inhibitors such as nivolumab, as well as combination trials with nivolumab plus ipilimumab.
BMS appears to only want the oxygen of publicity from positive data.
BMS (NYSE: BMY) is a company that has no problem issuing press releases about “positive data” – indeed, over the last two days alone they have already issued four so far to announce encouraging activity with nivolumab and ipilimumab in various tumour types. They are not so quick, however, to issue a press release to announce negative data.
Dr Oliver Sartor, the Laborde Professor of Cancer Research in the Medicine and Urology Departments of Tulane School of Medicine in New Orleans told BSB:
“Good news travels at lightning speed and bad news is never heard. This is something I learned many years ago when it comes to clinical trials.“
~ Dr Oliver Sartor
On clinicaltrials.gov the phase 3 trial of iplimumab in men with advanced prostate cancer prior to chemotherapy (NCT01057810) is simply shown as “completed” with “no study results posted” – see below:
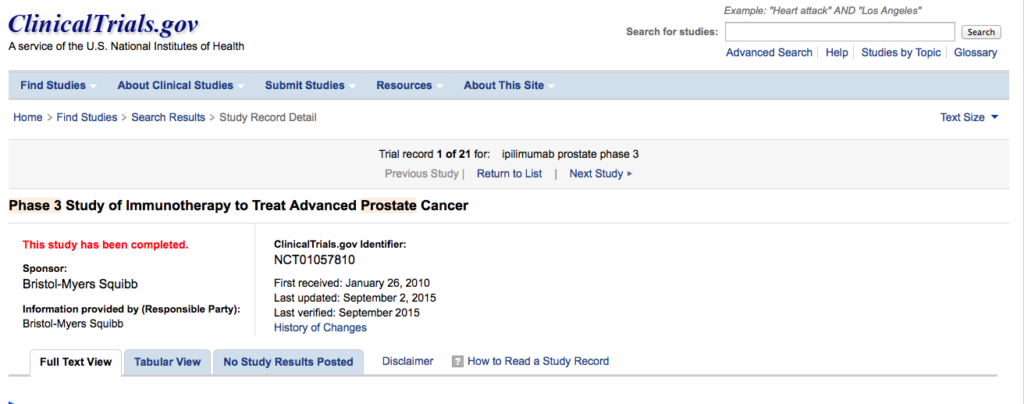
Over 600 men took at 134 centres around the world took part in the Randomized, Double-Blind, Phase 3 Trial to Compare the Efficacy of Ipilimumab vs Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naïve Castration Resistant Prostate Cancer.
It is, of course, possible that BMS may have told analysts or buried the news in some financial report, but if they did I missed it (update: yes, it was in the July 23, 2015 2Q 15 financial results press release).
Dr Sartor told me:
“I think around December I heard the news via the grapevine. It’s never been presented and it has gone underground. Apparently the sponsor does not want it to see the light of day, which is not appropriate.”
We all have negative trials and I cannot help but wonder if there was something else to the story. Was there some toxicity issues or other negative issues that were not aware of? Why is the data not being presented?
I can tell you that I have heard it is a negative trial but I’ve never seen anything in writing myself. I’ve never seen the written disclosure.”
Another global prostate cancer expert told me the same story, the trial “read out” last year and he was informed it was “negative” after ASCO 2015.
This thought leader told me that BMS have since shared no data, had not published the data at any meeting, or published the results. They were keeping the data to themselves, which did nothing to advance the field.
To clinical scientists and researchers at the ASCO annual meeting, the meeting theme of “Collective Wisdom” means sharing data about what works and what doesn’t work. Science moves forward by learning from experiments that work and those that don’t work. Often a negative result can be as important as a positive if it leads to greater understanding.
 The failure of BMS to disclose the results of a phase 3 study is a disservice to all who invested their time to make the trial happen, and lack of duty of care to all the men with advanced prostate cancer who participated, not only with the hope that they would live longer, but that they would contribute to research that might help others too.
The failure of BMS to disclose the results of a phase 3 study is a disservice to all who invested their time to make the trial happen, and lack of duty of care to all the men with advanced prostate cancer who participated, not only with the hope that they would live longer, but that they would contribute to research that might help others too.
It is inexcusable for a global company such as BMS to behave this way. If the data read out last year, the data could have been presented at the ASCO GU 2016 meeting, something that BMS did at ASCO GU 2013 when they presented the failure of their phase 3 prostate cancer trial with dasatinib.
Why was the pre-docetaxel ipilimumab trial negative?
On the Novel Targets podcast recorded at ASCO 2015 last year, Dr Sartor talked about the how close the post-docetaxel trial of ipilimumab in advanced prostate cancer came to being positive… but for the inclusion of men with visceral metastases, this trial would have been positive.
The ipi pre-chemo trial did not include patients with visceral mets, leading to the hope that it might be a positive trial if those men were excluded. Sadly, those hopes did not materialise.
Dr Sartor offered his clinical perspective:
“With regard to why it was negative, one can only speculate. The poor prognosis associated with visceral disease in the post-chemotherapy trial was a post hoc analysis.
Please remember that early in the post chemotherapy trial that more men died in the ipi arm compared to the comparison arm. It is conceivable that toxicity was to blame. This can be a toxic drug for some.
It is also possible that multiple lines of therapy given post protocol treatment contributed to a dilution of a positive affect. I believe this happened in the TAK 700 trial.
The bottom line is this drug was simply not active enough, or it was too toxic, or some combination of the above. Until we see more data it is hard to know. In the absence of data being presented, one might assume that toxicity played a role.”
Researchers in the field do need to know the reasons for the failure, especially given the interest that BMS have in combining ipilimumab with other cancer immunotherapy regimens, including those that do not have a high mutational load. Simply put, the data needs to be presented and published, it is unethical to do otherwise.
Update 1.35pm. Negative Trial mentioned in 2Q 2015 earnings release on July 23, 2015 – almost a year later the data remains unpublished!
Thanks to @AndyBiotech for drawing my attention to when the negative data was announced – in the 2Q 2015 earnings press release that went out on July 23, 2015. Link
“The company announced today that two Yervoy Phase 3 trials, Study -095 in metastatic castration-resistant prostate cancer and Study -156 in newly diagnosed extensive-stage disease small cell lung cancer, did not meet their primary endpoints of overall survival versus standard of care and have been discontinued. No new safety concerns with Yervoy were identified in either study. The company will complete a full evaluation of the data and work with investigators on the future publication of the results.”
If the trial had been positive or encouraging it would no doubt have merited a press release in its own right rather than a mere mention in a finance press release.
If a global prostate cancer expert, only heard from others in December (5 months after the news was buried in a financial press release) then the company has a problem in how it communicates with the prostate cancer community.
The point of this piece remains, that almost a year later the data remains unpublished and unpresented despite several opportunities to do so at major medical meetings such as ECCO 2015, ASCO GU 2016 and ASCO 2016.
If anyone from BMS wishes to advise when the data will be published or wants to dispute the accuracy of what two global prostate cancer thought leaders told me, they are welcome to send me a comment, and I will publish any response below.
Update Jun 6 BMS Promises to Publish
Thanks to Matt Herper (@MatthewHerper) from Forbes who used his influence to obtain a response from BMS to the question of where is the data? Do read his story on Forbes (link)
The company said they have plans to submit the data for publication in Q3 2016 – which probably means they are working on it now! Publication over a year after they announced the failure of their phase 3 ipilimumab trial in advanced prostate cancer is too long and reflects a double standard. The company wouldn’t wait 18 months to publish or present the data if it were positive; the same standard should apply either way.
As cancer immunotherapy moves forward into combinations, many companies are rushing from early signs of efficacy into large phase 3 trials in order to obtain a competitive advantage, with the hope of being first in that indication or beating others who have the potential to do a similar combination trial.
Only yesterday we saw early data presented in colorectal cancer (CRC) for atezolizumab (Roche/Genentech’s anti PD-L1 monoclonal antibody) in combination with a targeted therapy, the MEK inhibitor cobimetinib. Based on 4 partial responses (PRs) in a small group of patients with microsatellite stable disease (MSS), the company have initiated a phase 3 trial.
This trial has a lot of promise and potential, but it highlights how companies are moving fast on small amounts of data. Not every phase 3 trial is going to work in cancer immunotherapy. The field can’t wait 18 months for the publication of trial results when it does. The media need to write about failed trials too and hold companies accountable. What we do shouldn’t just be about eulogising the positive and new. While we all want to offer hope, at the same time we need to offer an honest reality check to the public.
Cancer immunotherapy still has a long way to go, not every person is going to respond and not every trial is going to work.
Dr Sartor would like to see the ipilimumuab prostate cancer data presented at a major medical meeting, and not just published in a journal:
I’m hopeful that we can learn from the trial even though it was negative. Results presented in a scientific form allow researchers to better understand the data. I certainly understand the financial implications and appreciate that Wall Street needs to know about negative data. Scientist have different needs. Every experiment has value and the fact that it was negative does not mean that we will not learn from it.
I honestly feel that the companies that run trials have an obligation to promptly report the results of both positive and negative trials in a scientific forum.
Why not submit the data for presentation at the European Society for Medical Oncology meeting in Copenhagen in October, for example?
Medical societies have no problem accepting abstracts, they recognise that debating and discussing why a trial failed to work can be as informative as discussing why something does. Meetings such as ASCO and ESMO should not just be for reporting positive data and an echo chamber for companies to share positive news.
I hope that BMS will present the data, as well as publish it. If the data was positive it would be at ESMO or another major medical meeting.
I’d like to thank Dr Sartor for being willing to share his views. Thanks also to Matt Herper for picking up the story and shining a spotlight on the topic. We’ll update the post with a link to the presentation or publication when it eventually comes out.
Update July 8: FDA set to fine companies $10K a day for late publication of clinical trial data
As reported by STAT news on June 29, Dr Francis Collins Director of the NIH announced that the FDA is set to crack down on companies and institutions that fail to report the results of clinical trial data in a timely manner. Link to Stat News story.
“Dr. Francis Collins, the director of the National Institutes of Health, later told reporters that a proposed rule — soon to become a final rule — should help by giving the agency more “clout” to crack down on institutions, not just individual investigators, when clinical trial data isn’t reported.
“That final rule is close to appearing,” he said. When it does, “we can basically say to Harvard, ‘Sorry, we’re not giving you any dollars until this principal investigator who ran a clinical trial deposits the data.’”
He also said the Food and Drug Administration will have the ability to impose $10,000-a-day fines on companies it regulates if they don’t comply with the reporting law.”
Quote from Stat News June 29 Story: “Biden threatens funding cuts for researchers who fail to report clinical trial results.“
While this blog post highlights one particular phase 3 trial prostate cancer immunotherapy trial, the bigger issue remains highly topical and is one companies, and those in PR should take note of: negative data needs to be reported in a timely manner.
Unless an exception or waiver is given by the Director of the NIH, my understanding of the current FDA regulations requires posting of the results of clinical trials to clinicaltrials.gov within 12 months of study completion. This is defined as the earlier of the estimated completion date of the trial or the actual completion date.
In practice this is not an onerous burden given the speed with which companies show they can generate presentations and publications when the data is positive!
The 12 month publication time is a statutory requirement set forth in Section 801 of the Food and Drug Administration Amendments Act (FDAAA 801).
As of the time of writing (July 8, 2016), BMS have yet to post any data for the failed phase 3 ipilimumab prostate cancer trial on clinicaltrials.gov. If the 12 months clock starts from the July 2015 actual completion date, then they are certainly taking it to the wire and run the risk of non-compliance, and potential penalities should the FDA seek to take enforcement action.
As outlined on clinicaltrials.gov website,
“FDAAA 801 establishes penalties for Responsible Parties who fail to comply with registration or results submission requirements. Penalties include civil monetary penalties and, for federally funded studies, the withholding of grant funds.”
Whether the FDA would seek to enforce the failure of a major company to comply with FDAAA801 remains to be seen, and BMS may yet deliver depending on what the actual completion date is and when that expires.
However if I was in corporate compliance, I don’t think I would be taking it to the wire or potentially playing with fire given that timely trial reporting is now a matter of public policy that has attracted the attention of politicians and regulators.
Both Vice President Biden and Dr Collins have sent an unequivocal message recently. Companies should take note and act accordingly, otherwise somebody will be fined, if only “pour encourager les autres.”
The prospect of an FDA rule with a $10,000 a day fine for late publication should certainly focus attention, if it hasn’t done so already.
Update August 23, 2016 Trial Results Posted on ClinicalTrials.Gov
On August 17, 2016 Bristol Myers finally posted the results of their phase 3 study of ipilimimumab to treat prostate cancer in the pre-chemotherapy setting. This was updated was processed on August 22 2016 by ClinicalTrials.gov. (link to results).
The primary completion date of the trial was April 2015, which means the 12 month data reporting clock started then, and according to Dr Deborah Zarin, Director of the National of Library Medicine, from her perspective the clock stops when the results are publicly available (note: this may change when new rules/regulations are published). Publicly posting data 16 months after the primary completion date is non-compliance by Bristol Myers; they get a D grade in my book. When you see the data, however, it is perhaps not surprising they were less than enthusiastic and chose to bury the initial announcement in an “earnings call” then finally post the data without annoucement in the dog days of summer.
When men with advanced prostate cancer are seeking treatment or considering a trial, they want to know the answer to the follow questions from their doctor, “if I take [fill in the blank}”:
- Will I live longer?
- Will I feel better?
The answer to both questions from the failed ipilimumab phase 3 trial is unfortunately, “No.”
Of the 837 men with advanced prostate cancer who were enrolled on this phase 3 trial, 602 were randomized and 598 were treated. 199 received placebo, 399 received ipilimumab 10mg/kg IV on days 1, 22, 43 and 64.
Median overall survival was 29.73 months in the placebo arm (26.12 to 34.17) compared to 28.65 months on ipilimumab (24.48 to 32.46).
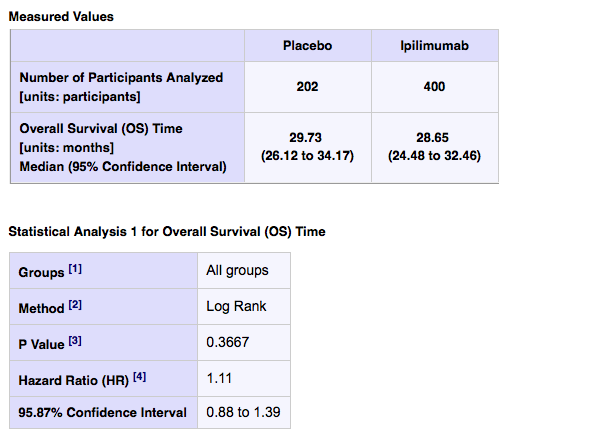
In other words, men receiving placebo did better. The hazard ratio was 1.11, which means they had an 11% higher risk of death if you started on ipi! What we don’t know is if there was a subset of men who may have benefited. More data is required.
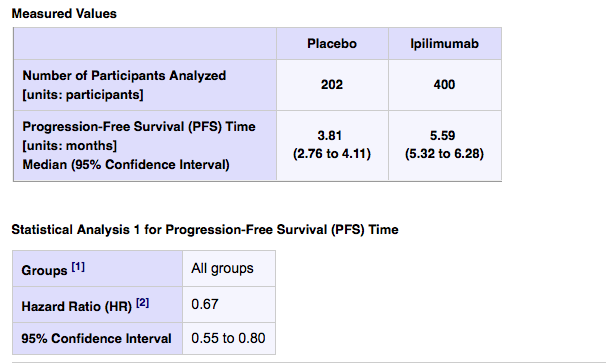
Progression free survival was slightly better on the ipi arm, with a median of 5.59 months (95% CI of 5.32 to 6.28) compared to a median of 3.81 months on placebo (95% CI of 2.76 to 4.11). We don’t know if this is statistically significant, but the favourable hazard ration of 0.67 suggests it most likely is.
Time to subsequent non-hormonal cytotoxic chemotherapy was 10.91 months (95% CI 8.44 to 14.59 months) in the placebo arm compared to 18.04 months in the Ipi arm (15.18 to 24.80). However, this delay to chemo did not translate into a better long term outcome for the IPI arm – the gold standard in cancer is how long do you live, which was the primary endpoint of the trial.
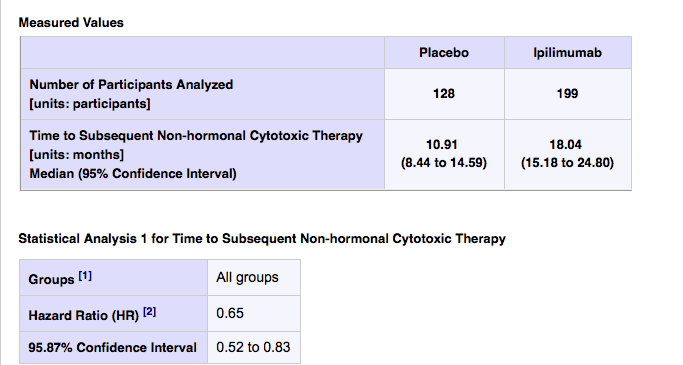
Overall, the OS data looks like it was the killer. We will no doubt learn more about the possible reasons for the negative overall survival when this data is published and/or presented.
It is an ethical requirement under the Declaration of Helsinki (7th Revision) that all clinical trial data be published:
“Negative and inconclusive as well as positive results should be published or otherwise made publicly available.”
It remains a disservice to the research and medical community, as well as the men who participated in this trial, to delay publication of data. Bristol Myers have offered no valid reason why it has taken 16 months for this data to be made publicly available.
Update Sept 19: Final Rule on Submission of Clinical Trial Data Published
Last Friday, September 16 saw the announcement of the long awaited final rule on what clinical trial data has to be supplied to clinicaltrials.gov, when it has to be provided and the penalties for non-compliance. The rule will be officially published in the Federal Register on September 21, 2016 and comes into effect in January 2017.
The final rule is extensive – it is 710 pages long, so something for lawyers and regulatory experts to get their teeth into. Here’s a link to the PDF If you want to read it.
There’s also more information on the NIH website, and an accompanying article was published in The New England Journal of Medicine (open access).

Learning why some combinations work and others don’t will be important to advance the field of cancer immunotherapy forwards. I do hope that companies and researchers will actively publish in a timely manner both positive and negative data. This is the final update to this post.



 Chicago – the ASCO 2016 annual meeting is in full swing. This is the third and last day of our rolling blog where we’re providing updates with top-line commentary throughout the data.
Chicago – the ASCO 2016 annual meeting is in full swing. This is the third and last day of our rolling blog where we’re providing updates with top-line commentary throughout the data. Chicago – at the 2016 annual meeting of the American Society of Clinical Oncology (ASCO), two global prostate cancer experts told me that a phase 3 prostate cancer immunotherapy trial in the pre-chemotherapy setting “read out” as negative last year, but they haven’t seen the data presented at a major medical meeting, published in a journal, and to the best of my knowledge, no press release has ever been issued.
Chicago – at the 2016 annual meeting of the American Society of Clinical Oncology (ASCO), two global prostate cancer experts told me that a phase 3 prostate cancer immunotherapy trial in the pre-chemotherapy setting “read out” as negative last year, but they haven’t seen the data presented at a major medical meeting, published in a journal, and to the best of my knowledge, no press release has ever been issued.
 The failure of BMS to disclose the results of a phase 3 study is a disservice to all who invested their time to make the trial happen, and lack of duty of care to all the men with advanced prostate cancer who participated, not only with the hope that they would live longer, but that they would contribute to research that might help others too.
The failure of BMS to disclose the results of a phase 3 study is a disservice to all who invested their time to make the trial happen, and lack of duty of care to all the men with advanced prostate cancer who participated, not only with the hope that they would live longer, but that they would contribute to research that might help others too.



 Chicago – it’s “Plenary Sunday” at the 2016 annual meeting of the American Society for Clinical Oncology (ASCO).
Chicago – it’s “Plenary Sunday” at the 2016 annual meeting of the American Society for Clinical Oncology (ASCO). Chicago – the annual meeting of the American Society for Clinical Oncology (Twitter #ASCO16) starts in earnest today, yesterday was primarily education sessions and a travel day for many.
Chicago – the annual meeting of the American Society for Clinical Oncology (Twitter #ASCO16) starts in earnest today, yesterday was primarily education sessions and a travel day for many.

