 “The Mannogram – Yes we scan” Jelle Barentsz, Professor of Radiology at Radbound University, Nijmegen, The Netherlands told the assembled media at the recent European Association of Urology (EAU) annual Congress in Paris.
“The Mannogram – Yes we scan” Jelle Barentsz, Professor of Radiology at Radbound University, Nijmegen, The Netherlands told the assembled media at the recent European Association of Urology (EAU) annual Congress in Paris.
Professor Barentsz described how advances in magnetic resonance imaging (MRI), and in particular multi-parametric MRI (Mp-MRI) offer the potential for the improved detection and characterization of prostate cancer.
In the same way there is a mammogram that women use for breast cancer screening, Professor Barentsz raised the possibility that using magnetic resonance imaging, men could have a mannogram to screen and diagnose prostate cancer.
 Some of the advantages of multi parametric MRI he highlighted include:
Some of the advantages of multi parametric MRI he highlighted include:
- Prediction of tumor aggression
- Prediction of low vs intermediate or high grade prostate cancer correctly in 95% of men in a trial as compared to 54% with TRUS (trans-rectal ultrasound guided) biopsy
- In cases where there was a negative TRUS biopsy initially, mp-MRI and MR guided biopsy detected prostate cancer in 41% (108/265 of trial participants), with 87% of the prostate cancer detected being significant.
It is beyond the scope of this post to go into the physics of multi-parametric MRI or discuss in more detail the imaging trial data on which the above conclusions are based. However, for those interested in this area, I have included details of some of the references Professor Barentsz kindly provided at the end of the post.
Why do we need new techniques for prostate cancer diagnosis?
During their lifetime 1 in 6 men will be clinically diagnosed with prostate cancer. There are 899,000 new cases and 258,000 deaths per year in Europe.
The current diagnostic tools of digital rectal examination (DRE), serum prostate specific antigen (PSA) and trans-rectal ultrasound guided (TRUS) biopsy have a number of limitations for prostate cancer detection.
This includes a lack of specificity (PSA = 36%), insensitivity (DRE = 37%) or failure to detect cancer due to sampling error with TRUS biopsy (more than 20% of cancers are not detected on first biopsy).
The result is that we end up with large numbers of men with elevated men or rising PSA, who have repeat biopsies. This comes at a high cost to health care providers, the uncertainty of diagnosis and the discomfort that comes with a TRUS biopsy (TRUS-Bx).
Many men undergo diagnostic procedures only to find they don’t have prostate cancer. One of the key issues in the prostate cancer screening debate is this unacceptable harm/benefit ratio.
Prostate biopsy to confirm cancer diagnosis is an invasive procedure as Professor Jenny Donovan, Head of the School of Social and Community Medicine, at the University of Bristol told the EAU Congress.
 In a plenary session, Prof Donovan described some of the initial findings from the PROBE (Prostate Biopsy Effects study), which looked at 1,147 men who received a TRUS-Bx.
In a plenary session, Prof Donovan described some of the initial findings from the PROBE (Prostate Biopsy Effects study), which looked at 1,147 men who received a TRUS-Bx.
“The majority of men tolerated biopsy reasonably well – over 60% experience minor symptoms. However, around one third experience symptoms that bothered them,” she said.
Professor Donovan went on to give examples of some of the feedback on the biopsy experience that researchers obtained:
“I found it incredibly painful and distressing – biopsy with a nail gun,” one man in the PROBE study said.
Data presented by Professor Donovan showed that after the procedure, 11% of men would not want another biopsy (this rose to 20%) seven days later.
Magnetic Resonance Imaging Guided Biopsy may offer benefits
Given the discomfort that many men experience with TRUS biopsy, and the fact a negative biopsy does not automatically mean no cancer (there’s a risk of sampling error), the use of MR guided biopsy may offer significant benefits.
One is that instead of 12 cores being sampled by the TRUS-Bx, only 2 cores are obtained using the MR guided biopsy technique that Professor Barentsz described.
Personally, I would prefer to have two precise cores samples taken from me through imaged based guidance, than have a TRUS biopsy that is like a nail gun that shoots in 12 sampling rods into the target area of the prostate. This would be like the difference between a sniper rifle and a blunderbuss, metaphorically.
One of the practice implications of this is that radiologists may end up performing MR guided biopsies instead of urologists performing TRUS biopsies.
Urologists in private practice or those who are paid per procedure may not be happy about the practice changing implications that may result.
The MR imaging research that Professor Barentsz described at EAU is not without its limitations and one concern is the reproducibility of advanced imaging techniques outside of an expert university or academic setting. I put this question to Professor Barentsz in the media briefing and have included an excerpt of his reply that you can listen to:
 According to Professor Barentsz, the imaging techniques for MR guided prostate biopsy are readily reproducible outside the academic environment with guidelines already in place for standardized acquisition protocols and structured interpretation of results.
According to Professor Barentsz, the imaging techniques for MR guided prostate biopsy are readily reproducible outside the academic environment with guidelines already in place for standardized acquisition protocols and structured interpretation of results.
“We are now at the point that the standardization of prostate MRI as well as the standardization of reporting with all kind of computer assistance is a fact and not fiction anymore,” said Barentsz.
The European Society of Urogenital Radiology (ESUR) recently published MR guidelines, that include a structured reporting system, called PI-RADS (prostate imaging, reporting, and data system). This has been adopted in the United States by the American College of Radiology (ACR).
Before men with prostate cancer can expect to see these advanced imaging techniques used outside of expert centers, however, urologists and radiologists will need to agree on the benefits they offer and adapt their practice accordingly. Urologists may be reluctant to move away from TRUS biopsy, so it is likely widespread implementation will take some time and require education, and explanation of the evidence based medicine that supports the proposed change in practice.
The conclusion from Professor Barentsz’s EAU presentation is that imaging looks likely to play an increasing role in the diagnosis of prostate cancer.
It will be an exciting area to watch. The idea of a Mannogram has the potential to become a reality that would benefit the 1 in 6 men who will be diagnosed with Prostate Cancer during their lifetime.
References
 Hambrock, T., Somford, D., Huisman, H., van Oort, I., Witjes, J., Hulsbergen-van de Kaa, C., Scheenen, T., & Barentsz, J. (2011). Relationship between Apparent Diffusion Coefficients at 3.0-T MR Imaging and Gleason Grade in Peripheral Zone Prostate Cancer Radiology, 259 (2), 453-461 DOI: 10.1148/radiol.11091409
Hambrock, T., Somford, D., Huisman, H., van Oort, I., Witjes, J., Hulsbergen-van de Kaa, C., Scheenen, T., & Barentsz, J. (2011). Relationship between Apparent Diffusion Coefficients at 3.0-T MR Imaging and Gleason Grade in Peripheral Zone Prostate Cancer Radiology, 259 (2), 453-461 DOI: 10.1148/radiol.11091409
Hoeks, C., Schouten, M., Bomers, J., Hoogendoorn, S., Hulsbergen-van de Kaa, C., Hambrock, T., Vergunst, H., Sedelaar, J., Fütterer, J., & Barentsz, J. (2012). Three-Tesla Magnetic Resonance–Guided Prostate Biopsy in Men With Increased Prostate-Specific Antigen and Repeated, Negative, Random, Systematic, Transrectal Ultrasound Biopsies: Detection of Clinically Significant Prostate Cancers European Urology DOI: 10.1016/j.eururo.2012.01.047
Barentsz, J., Dickinson, L., & Sciarra, A. (2011). Re: Axel Heidenreich. Consensus Criteria for the Use of Magnetic Resonance Imaging in the Diagnosis and Staging of Prostate Cancer: Not Ready for Routine Use. Eur Urol 2011;59:495–7 European Urology, 60 (1) DOI: 10.1016/j.eururo.2011.03.013
Hambrock, T., Hoeks, C., Hulsbergen-van de Kaa, C., Scheenen, T., Fütterer, J., Bouwense, S., van Oort, I., Schröder, F., Huisman, H., & Barentsz, J. (2012). Prospective Assessment of Prostate Cancer Aggressiveness Using 3-T Diffusion-Weighted Magnetic Resonance Imaging–Guided Biopsies Versus a Systematic 10-Core Transrectal Ultrasound Prostate Biopsy Cohort European Urology, 61 (1), 177-184 DOI: 10.1016/j.eururo.2011.08.042
Barentsz, J., Richenberg, J., Clements, R., Choyke, P., Verma, S., Villeirs, G., Rouviere, O., Logager, V., & Fütterer, J. (2012). ESUR prostate MR guidelines 2012 European Radiology, 22 (4), 746-757 DOI: 10.1007/s00330-011-2377-y
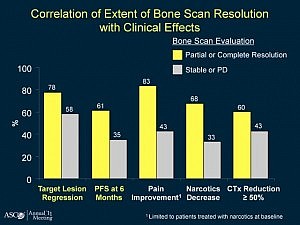
 he results of the phase 3 clinical trial of dasatinib (Sprycel) plus docetaxel/prednisone versus placebo and docetaxel/prednisone in men with castration-resistant metastatic prostate cancer (CRPC) are expected soon.
he results of the phase 3 clinical trial of dasatinib (Sprycel) plus docetaxel/prednisone versus placebo and docetaxel/prednisone in men with castration-resistant metastatic prostate cancer (CRPC) are expected soon.


 As Sally Church, PhD
As Sally Church, PhD 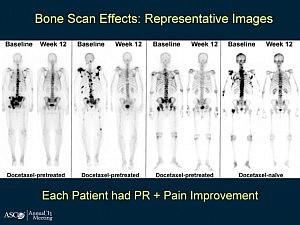


 “The Mannogram – Yes we scan” Jelle Barentsz, Professor of Radiology at Radbound University, Nijmegen, The Netherlands told the assembled media at the recent European Association of Urology (EAU) annual Congress in Paris.
“The Mannogram – Yes we scan” Jelle Barentsz, Professor of Radiology at Radbound University, Nijmegen, The Netherlands told the assembled media at the recent European Association of Urology (EAU) annual Congress in Paris. Some of the advantages of multi parametric MRI he highlighted include:
Some of the advantages of multi parametric MRI he highlighted include: In a plenary session, Prof Donovan described some of the initial findings from the PROBE (Prostate Biopsy Effects study), which looked at 1,147 men who received a TRUS-Bx.
In a plenary session, Prof Donovan described some of the initial findings from the PROBE (Prostate Biopsy Effects study), which looked at 1,147 men who received a TRUS-Bx. According to Professor Barentsz, the imaging techniques for MR guided prostate biopsy are readily reproducible outside the academic environment with guidelines already in place for standardized acquisition protocols and structured interpretation of results.
According to Professor Barentsz, the imaging techniques for MR guided prostate biopsy are readily reproducible outside the academic environment with guidelines already in place for standardized acquisition protocols and structured interpretation of results.