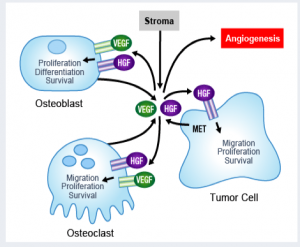
The recent announcement from the United States Preventative Services Task Force (USPSTF) on prostate cancer screening has stimulated a lot of debate. “Dr Len’s Cancer Blog” from the American Cancer Society has a thoughtful piece about the “to screen or not to screen” dilemma. It is reminiscent of the breast cancer mammography debate.
Scott Hensley’s post on “Shots”, the NPR health blog also provides a good overview of the debate and issues. I confess I was surprised by the vehemence of the response from some practising urologists on twitter such as Dr Benjamin J. Davies (@daviesbj), Assistant Professor of Urology at the University of Pittsburgh School of Medicine:

What do the US Preventative Task Force authors say? In the findings published in the October 7, 2011 issue of the Annals of Internal Medicine, the conclusion from their peer-review of the literature is that:
“screening based on PSA identifies additional prostate cancers, but most trials found no statistically significant effect on prostate cancer–specific mortality.”
This is nothing new – the evidence in favor of Prostate-Specific Antigen (PSA) screening for prostate cancer has for sometime been inconclusive at best. Research published in the British Medical Journal earlier this year suggested that indiscriminate screening for prostate cancer may not be of value until we obtain more sensitive biomarkers.
All the USPSTF have done is a retrospective literature review that confirms our current state of knowledge.
Despite the amount of $$$ that have been spent on PSA clinical studies, no convincing data in support of it as a screening tool for all men has been developed. That’s not to say that it has no utility or benefit.
If PSA screening does detect some prostate cancers and early treatment may save lives particularly in those with an aggressive form of the disease, then it’s hard not ask:
- What is the alternative if PSA screening is now discouraged?
- Will this mean more men will not be screened, even those at high risk, with a consequent increase in prostate cancer mortality or lower overall survival?
- Will insurers refuse to cover PSA testing moving forwards?
- Will family physicians actively discourage PSA testing?
One limitation of the USPSTF report that struck me while reading through the analysis and recommendations, was the absence of data from the Prostate Cancer Intervention versus Observation (PIVOT) trial that was presented in the plenary session at the American Urological Association (AUA) annual meeting earlier this year.
I wrote in a previous blog post about the PIVOT data presented at AUA 2011 by Dr Wilt. This VA/NCI/AHRQ randomized clinical trial showed in a 12 year follow-up of over 5000 men with localized prostate cancer, no benefit from radical prostatectomy in low risk early stage patients.
In my opinion it is practice changing data that sadly has not yet been published in a peer-reviewed journal which is why it was not included in the USPSTF analysis. A few photographs that I took at the presentation of the PIVOT data are shown below:
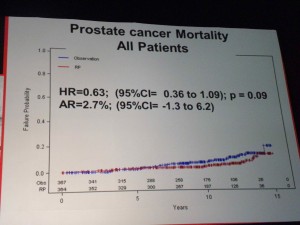
The 12 year survival data showed that in those patients with PSA <=10 ng/ml there was no significant difference in survival over observation versus RP.
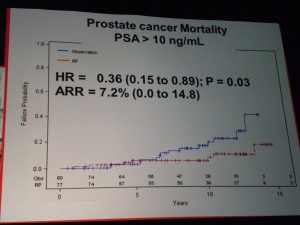
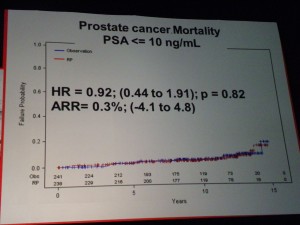 However in those patients with PSA > 10ng/ml there was a significant difference in mortality (p=0.03 HR=0.36) suggesting that in the small sub-group of patients with high PSA, RP did provide a benefit over “watchful waiting.” PSA measurements may therefore help in the treatment and ongoing management of patients.
However in those patients with PSA > 10ng/ml there was a significant difference in mortality (p=0.03 HR=0.36) suggesting that in the small sub-group of patients with high PSA, RP did provide a benefit over “watchful waiting.” PSA measurements may therefore help in the treatment and ongoing management of patients.
 If one of the challenges of PSA screening is the issue of the harms from treatment, then the PIVOT data that showed no difference in mortality through watchful waiting over radical prostatectomy (RP) except for high risk patients is important.
If one of the challenges of PSA screening is the issue of the harms from treatment, then the PIVOT data that showed no difference in mortality through watchful waiting over radical prostatectomy (RP) except for high risk patients is important.
Roger Chou and his colleagues in their Annals paper did note this:
“When available, results from the Prostate Cancer Intervention Versus Observation Trial, which compared prostatectomy with watchful waiting for screening-detected cancer, may help clarify which patients would benefit from prostatectomy or other active treatments, potentially reducing harms from unnecessary treatment.”
The issue may, therefore, not be one that PSA screening per-se is the problem, but that in too many men, a raised PSA results in unnecessary surgery or overtreatment with its consequent risks and harms.
As reported by Alemozaffar and colleagues in the September 21, 2011 issue of the Journal of the American Medical Association (JAMA), 2 years after RP only 35% of men could maintain a functional erection.
“At 2 years after treatment, erectile dysfunction was reported by 619 of 987 (63% [95% CI, 60%-66%]) men (334/511 [65% {95% CI, 61%- 69%}] in the prostatectomy group”
That’s depressing news when coupled with other side-effects from RP or other interventions such as incontinence or problems with bowel function.
It adds further support to the idea that the potential risks versus benefits from prostate cancer surgery and deciding who should actually have surgery or other intervention, is where there’s also a need for more debate.
Men need to be able to make an informed decision. Some urologists may not be unbiased in their treatment recommendations given they have a vested interest in earning money from surgery or maintaining utilization rates of expensive robotics. Data from the PIVOT trial, when published, is evidence-based medicine that will impact their practice.
Use of prostate cancer risk calculators to assess those patients at high risk who might benefit from RP or other intervention could also help lower the harms that currently impact the utility of PSA screening.
How will men approach the PSA screening debate? Despite the USPSTF recommendations I will still be asking my physician for an annual PSA test, so long as it remains covered by my health insurance. The PSA test and digital rectal exam remain the main ways we have to detect prostate cancer early.
Psychologically I value the risk of picking up a disease early higher than the downside of a false positive. I would, however, not rush into any surgery or radiotherapy absent a clear finding that the benefits of intervention outweighed the potential harm.
My approach towards prostate cancer PSA screening again reminds me of the debate over mammography. We all want to discover cancer early, and have the best possible treatment options as a result.
Hopefully, as our understanding of the biology of prostate cancers and associated biomarkers develop we may be able to generate new screening tests that have fewer false positives than PSA and provide for more accurate early diagnosis.
 Alemozaffar, M., Regan, M., Cooperberg, M., Wei, J., Michalski, J., Sandler, H., Hembroff, L., Sadetsky, N., Saigal, C., Litwin, M., Klein, E., Kibel, A., Hamstra, D., Pisters, L., Kuban, D., Kaplan, I., Wood, D., Ciezki, J., Dunn, R., Carroll, P., & Sanda, M. (2011). Prediction of Erectile Function Following Treatment for Prostate Cancer JAMA: The Journal of the American Medical Association, 306 (11), 1205-1214 DOI: 10.1001/jama.2011.1333
Alemozaffar, M., Regan, M., Cooperberg, M., Wei, J., Michalski, J., Sandler, H., Hembroff, L., Sadetsky, N., Saigal, C., Litwin, M., Klein, E., Kibel, A., Hamstra, D., Pisters, L., Kuban, D., Kaplan, I., Wood, D., Ciezki, J., Dunn, R., Carroll, P., & Sanda, M. (2011). Prediction of Erectile Function Following Treatment for Prostate Cancer JAMA: The Journal of the American Medical Association, 306 (11), 1205-1214 DOI: 10.1001/jama.2011.1333
Roger Chou, MD, Jennifer M. Croswell, MD, MPH, Tracy Dana, MLS, Christina Bougatsos, BS, Ian Blazina, MPH, Rongwei Fu, PhD, Ken Gleitsmann, MD, MPH, Helen C. Koenig, MD, MPH, Clarence Lam, MD, MPH, Ashley Maltz, MD, MPH, J. Bruin Rugge, MD, MPH, & Kenneth Lin, MD (2011). Screening for Prostate Cancer: A Review of the Evidence for the U.S. Preventive Services Task Force Annals of Internal Medicine, E-375 (October 7)



 However in those patients with PSA > 10ng/ml there was a significant difference in mortality (p=0.03 HR=0.36) suggesting that in the small sub-group of patients with high PSA, RP did provide a benefit over “watchful waiting.” PSA measurements may therefore help in the treatment and ongoing management of patients.
However in those patients with PSA > 10ng/ml there was a significant difference in mortality (p=0.03 HR=0.36) suggesting that in the small sub-group of patients with high PSA, RP did provide a benefit over “watchful waiting.” PSA measurements may therefore help in the treatment and ongoing management of patients. If one of the challenges of PSA screening is the issue of the harms from treatment, then the PIVOT data that showed no difference in mortality through watchful waiting over radical prostatectomy (RP) except for high risk patients is important.
If one of the challenges of PSA screening is the issue of the harms from treatment, then the PIVOT data that showed no difference in mortality through watchful waiting over radical prostatectomy (RP) except for high risk patients is important.
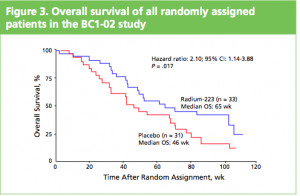 At the ASCO 2011 meeting in Chicago there was
At the ASCO 2011 meeting in Chicago there was  What makes Alpharadin exciting as a new treatment option for castration resistant prostate cancer (CRPC) is that the ALSYMPCA trial data shows that it not only provides a significant median overall survival (OS) benefit of 2.8 months compared to placebo (14 months versus 11.2 months, p=0.00185, HR 0.695), but significantly delays the time to first skeletal event by 5.2 months (13.6 months versus 8.4 months, p=0.00046, HR 0.610).
What makes Alpharadin exciting as a new treatment option for castration resistant prostate cancer (CRPC) is that the ALSYMPCA trial data shows that it not only provides a significant median overall survival (OS) benefit of 2.8 months compared to placebo (14 months versus 11.2 months, p=0.00185, HR 0.695), but significantly delays the time to first skeletal event by 5.2 months (13.6 months versus 8.4 months, p=0.00046, HR 0.610). At the press briefing late friday afternoon in Stockholm, Dr Chris Parker of the Royal Marsden Hospital and PI of the ALSYMPCA study said that “Radium-223, a novel alpha-pharmaceutical, may provide a new standard of care for the treatment of CRPC patients with bone metastases.”
At the press briefing late friday afternoon in Stockholm, Dr Chris Parker of the Royal Marsden Hospital and PI of the ALSYMPCA study said that “Radium-223, a novel alpha-pharmaceutical, may provide a new standard of care for the treatment of CRPC patients with bone metastases.”
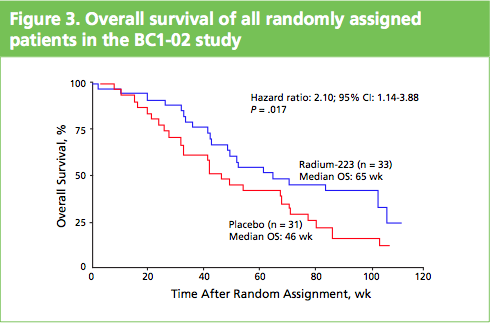
 The market for prostate cancer therapies is set to expand from $1 billion currently to $5 billion by 2015, according to analysts reported by
The market for prostate cancer therapies is set to expand from $1 billion currently to $5 billion by 2015, according to analysts reported by