Despite promising preclinical data suggesting that selenium and vitamin E may reduce prostate cancer risk, a randomized trial started in 2001 with over 35,000 men now suggests otherwise.
Given the prevalence of people taking vitamin supplements, these findings have important public health implications.
The results of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) reported in the October 12, 2011 issue of the Journal of the American Medical Association (JAMA) show that dietary supplementation with Vitamin E significantly increased the risk of prostate cancer in healthy men.
Over 35,000 men were randomized into 4 groups: selenium (200 mg/d from L-selenomethionine) with matching placebo, vitamin E (400IU/d of all rac-a-tocopherol acetate) with matching placebo, both agents, or placebo. The study was stopped in September 2008 as a result of an interim analysis that showed lack of efficacy for risk reduction and futility analysis showed a lack of future benefit.
The data in 2008 with a median follow-up of 5.5 years suggested an increased risk of prostate cancer observed with vitamin E. That finding has now reached statistical significance in the latest analysis of the trial data, 7 years after the last patient was randomized.
Participants were healthy men at average risk of prostate cancer. They were monitored every 6 months and recommended to undergo prostate-specific antigen (PSA) and digital rectal examination (DRE) based on the standard of care in their community. The primary end point was prostate cancer incidence resulting from routine community care.
The updated results from the SELECT trial are published in JAMA by lead author Eric Klein from the Cleveland Clinic and colleagues. The data shows that the incidence of prostate cancer was greater in all treatment groups compared to placebo, but statistically significant only in the vitamin E group alone (P=0.008, HR: 1.17; 99% CI, 1.004-1.36).
The authors note this data means:
“The risk of prostate cancer at 7 years of median follow-up was increased by 17% in men randomized to supplementation with vitamin E alone, a difference that started to appear about 3 years after randomization.”
Why does Vitamin E supplementation cause this increased risk? The authors in their JAMA paper make no suggestion.
The data from this trial has important implications for all men who take multivitamin supplements. The authors note that more than 50% of all individuals over 60 take a vitamin supplement and 23% of them take a 400 IU/d or greater dose of Vitamin E, a dose that now has been shown to increase the risk of prostate cancer.
All too often we associate taking vitamins as healthy living. The conclusion of the authors is one that we should all take note of:
“The observed 17% increase in prostate cancer incidence demonstrates the potential for seemingly innocuous yet biologically active substances such as vitamins to cause harm.”
Only by doing clinical trials such as SELECT, can we assess the true harms and benefits of unregulated over-the-counter products such as vitamins.
 Eric A. Klein, MD, Ian M. Thompson Jr, MD, Catherine M. Tangen, DrPH, John J. Crowley, PhD, M. Scott Lucia, MD, Phyllis J. Goodman, MS, Lori M. Minasian, MD, Leslie G. Ford, MD, Howard L. Parnes, MD, J. Michael Gaziano, MD, MPH, Daniel D. Karp, MD, Michael M. Lieber, MD, Philip J. Walther, MD, PhD, Laurence Klotz, MD, J. Kellogg Parsons, MD, MHS, Joseph L. Chin, MD, Amy K. Darke, MS, Scott M. Lippman, MD, Gary E. Goodman, MD, Frank L. Meyskens Jr, MD, & Laurence H. Baker, DO (2011). Vitamin E and the Risk of Prostate Cancer, The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA: The Journal of the American Medical Association, 306 (14), 1549-1556
Eric A. Klein, MD, Ian M. Thompson Jr, MD, Catherine M. Tangen, DrPH, John J. Crowley, PhD, M. Scott Lucia, MD, Phyllis J. Goodman, MS, Lori M. Minasian, MD, Leslie G. Ford, MD, Howard L. Parnes, MD, J. Michael Gaziano, MD, MPH, Daniel D. Karp, MD, Michael M. Lieber, MD, Philip J. Walther, MD, PhD, Laurence Klotz, MD, J. Kellogg Parsons, MD, MHS, Joseph L. Chin, MD, Amy K. Darke, MS, Scott M. Lippman, MD, Gary E. Goodman, MD, Frank L. Meyskens Jr, MD, & Laurence H. Baker, DO (2011). Vitamin E and the Risk of Prostate Cancer, The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA: The Journal of the American Medical Association, 306 (14), 1549-1556
 The
The  After the recent JP Morgan Healthcare conference, San Francisco remains the destination of choice for forthcoming medical meetings.
After the recent JP Morgan Healthcare conference, San Francisco remains the destination of choice for forthcoming medical meetings.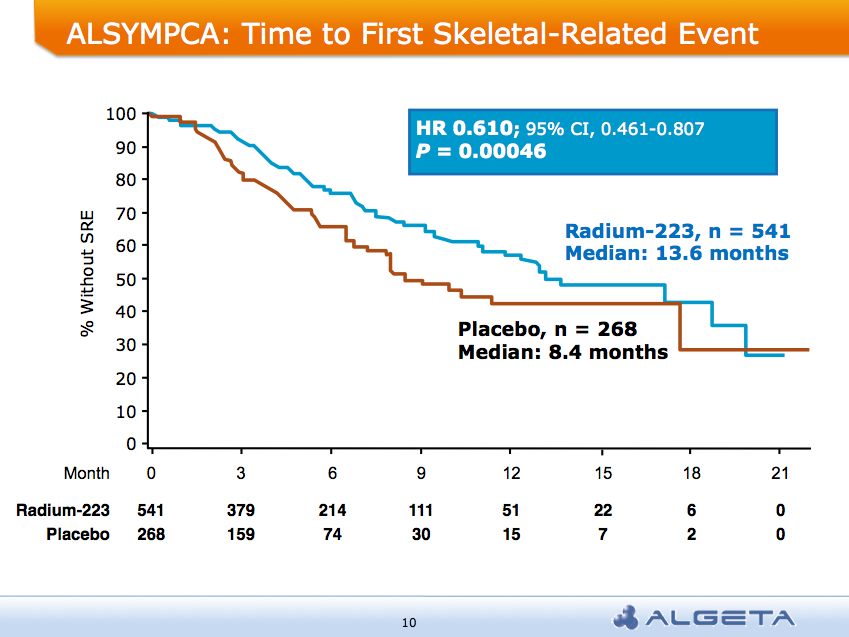 AND a median overall survival of 14 months compared to 11.2 months for placebo group:
AND a median overall survival of 14 months compared to 11.2 months for placebo group: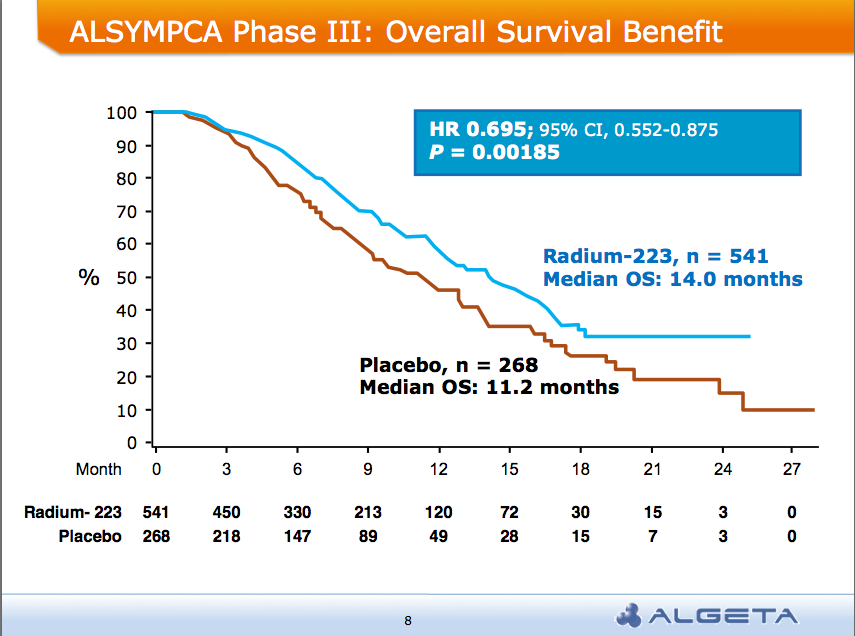 Alpharadin is on the fast track to FDA approval this year
Alpharadin is on the fast track to FDA approval this year This morning the 8am session at the
This morning the 8am session at the  The highlight, in my opinion, was Oliver Sartor’s excellent presentation on radium-223 chloride (Alpharadin) in which he cogently outlined its mechanism of action. He explained that radium-223:
The highlight, in my opinion, was Oliver Sartor’s excellent presentation on radium-223 chloride (Alpharadin) in which he cogently outlined its mechanism of action. He explained that radium-223:

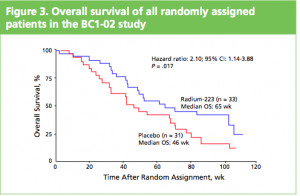 At the ASCO 2011 meeting in Chicago there was
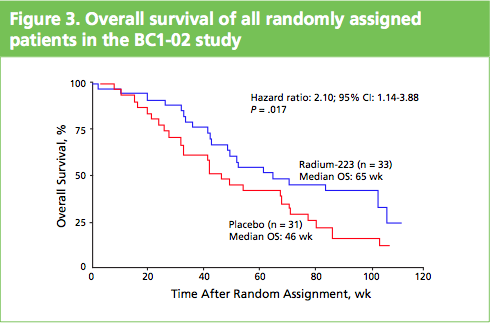
At the ASCO 2011 meeting in Chicago there was  What makes Alpharadin exciting as a new treatment option for castration resistant prostate cancer (CRPC) is that the ALSYMPCA trial data shows that it not only provides a significant median overall survival (OS) benefit of 2.8 months compared to placebo (14 months versus 11.2 months, p=0.00185, HR 0.695), but significantly delays the time to first skeletal event by 5.2 months (13.6 months versus 8.4 months, p=0.00046, HR 0.610).
What makes Alpharadin exciting as a new treatment option for castration resistant prostate cancer (CRPC) is that the ALSYMPCA trial data shows that it not only provides a significant median overall survival (OS) benefit of 2.8 months compared to placebo (14 months versus 11.2 months, p=0.00185, HR 0.695), but significantly delays the time to first skeletal event by 5.2 months (13.6 months versus 8.4 months, p=0.00046, HR 0.610). At the press briefing late friday afternoon in Stockholm, Dr Chris Parker of the Royal Marsden Hospital and PI of the ALSYMPCA study said that “Radium-223, a novel alpha-pharmaceutical, may provide a new standard of care for the treatment of CRPC patients with bone metastases.”
At the press briefing late friday afternoon in Stockholm, Dr Chris Parker of the Royal Marsden Hospital and PI of the ALSYMPCA study said that “Radium-223, a novel alpha-pharmaceutical, may provide a new standard of care for the treatment of CRPC patients with bone metastases.”