Prostate Cancer Market to expand from $1B to $5B by 2015
 The market for prostate cancer therapies is set to expand from $1 billion currently to $5 billion by 2015, according to analysts reported by this morning’s Washington Post/Bloomberg news. This is perhaps no surprise given the recent approval of abiraterone acetate (Zytiga®) from Ortho Biotech (JNJ).
The market for prostate cancer therapies is set to expand from $1 billion currently to $5 billion by 2015, according to analysts reported by this morning’s Washington Post/Bloomberg news. This is perhaps no surprise given the recent approval of abiraterone acetate (Zytiga®) from Ortho Biotech (JNJ).
New clinical data on prostate cancer clinical trial results is expected at the 2011 annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago this weekend from many of the prostate cancer therapies in development such as MDV3100, TAK700, ARN-509, cabozantinib (XL184), ipilimumab, custirsen (OGX-11), BPX-101, alpharadin, denosumab (Xgeva®) and Prostvac-VF.
Indeed, one could argue that prostate cancer is becoming a competitive marketplace. Any emerging biotechnology company that is not already developing a prostate cancer drug is likely to find it a hard market in which to create a blockbuster. By the time any drug comes to market, there will be incumbents with effective products who have captured market share.
Prostate cancer is an exciting market to watch from a marketing strategy and patient perspective, as several companies potentially bring new products to market over the next few years.
However, the bottom line is that patients will live longer as a result of all the innovation that is taking place. Not only that but physician education and awareness of how to treat this disease is also likely to improve as they seek out knowledge on new therapies and treatments. This to many will make a major difference. At the recent American Urological Association (AUA) annual meeting, the sessions on treatment of prostate cancer were standing room only. There is clearly a demand for knowledge out there as the treatment paradigms change.
At the other end of the spectrum, there is also innovation taking place in terms of improved diagnosis and treatment of prostate cancer. Whether we should screen all men for PSA remains a controversial topic, although use of risk calculators do appear to offer less false positives. Indeed, calculating risk is going to be one of the key areas that primary care physicians and urologists need to focus on, particularly in the light of the PIVOT trial data that was presented at AUA, showing radical prostatectomy (with risks including incontinence and erectile dysfunction) was not better than watchful waiting in low-risk, early stage disease.
However, a presentation I am looking forward to at ASCO 2011 is on circulating tumor cells (CTC) and whether these can be a prognostic or even a predictive biomarker. Both the phase III MDV3100 and abiraterone acetate clinical trials captured CTC data. It will be exciting news at ASCO 2011 if circulating tumor cells that require only a blood sample offer an improvement over PSA not only for detection of prostate cancer, but in monitoring the disease over time.
I will be at ASCO 2011 this weekend, and look forward to writing more on prostate cancer from the conference!
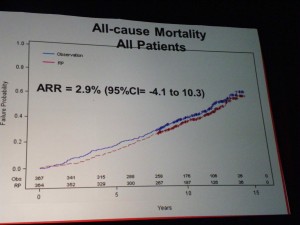 All-Cause Mortality
All-Cause Mortality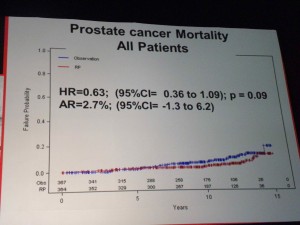 Prostate Cancer Mortality – all patients
Prostate Cancer Mortality – all patients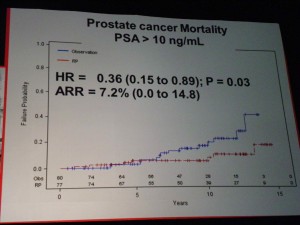 Only in the high-risk groups (PSA>10) was there a significant benefit to RP, in terms of lowering Prostate Cancer Mortality.
Only in the high-risk groups (PSA>10) was there a significant benefit to RP, in terms of lowering Prostate Cancer Mortality.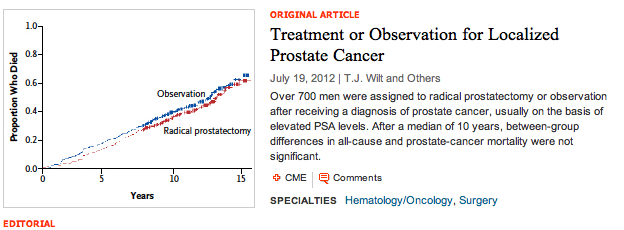
 One of the sessions that I attended at the 2011 annual meeting of the American Urological Association (AUA) focused on research into advanced prostate cancer. A particularly thought provoking presentation was:
One of the sessions that I attended at the 2011 annual meeting of the American Urological Association (AUA) focused on research into advanced prostate cancer. A particularly thought provoking presentation was: Presented by Alex Haese from Hamburg, Germany, this paper was a retrospective analysis of 1,574 patients who had a biochemical recurrence (PSA > 0.2 mg/dl) following RP. Researchers looked at clinical progression and cancer specific survival rates and compared their findings to published United States data.
Presented by Alex Haese from Hamburg, Germany, this paper was a retrospective analysis of 1,574 patients who had a biochemical recurrence (PSA > 0.2 mg/dl) following RP. Researchers looked at clinical progression and cancer specific survival rates and compared their findings to published United States data. There is a lot of focus at the
There is a lot of focus at the 


