PARP inhibition in metastatic prostate cancer

Who’s King of the PARP castle?
After yesterday’s review and expert commentary on the phase 3 PROfound trial presented in the Presidential Session at ESMO 2019, we’re continuing our look at PARP inhibitors in advanced prostate cancer.
Perhaps surprisingly, there were a lot of insights to be found in the posters that were presented and discussed at the meeting for other PARPs in clinical development.
How do these stack up against olaparib? We’re not fans of cross-trial comparisons as they always come with a mandatory health warning, but if you want to consider the emerging landscape, it is important to be aware of the different patient populations, lines of therapy, and details of the trial designs.
For additional perspective at ESMO19, we spoke to a European prostate cancer expert who kindly talked about his clinical practice and also offered insights into a PARP clinical trial he and colleagues presented in Barcelona.
Who will be King of the PARP castle in advanced prostate cancer?
To learn more from our latest oncology conference insights and get a heads up on our latest post ESMO Coverage and reflections, including a specialist thought leader interview, subscribers can log-in or you can click to gain access to BSB Premium Content.
This content is restricted to subscribers







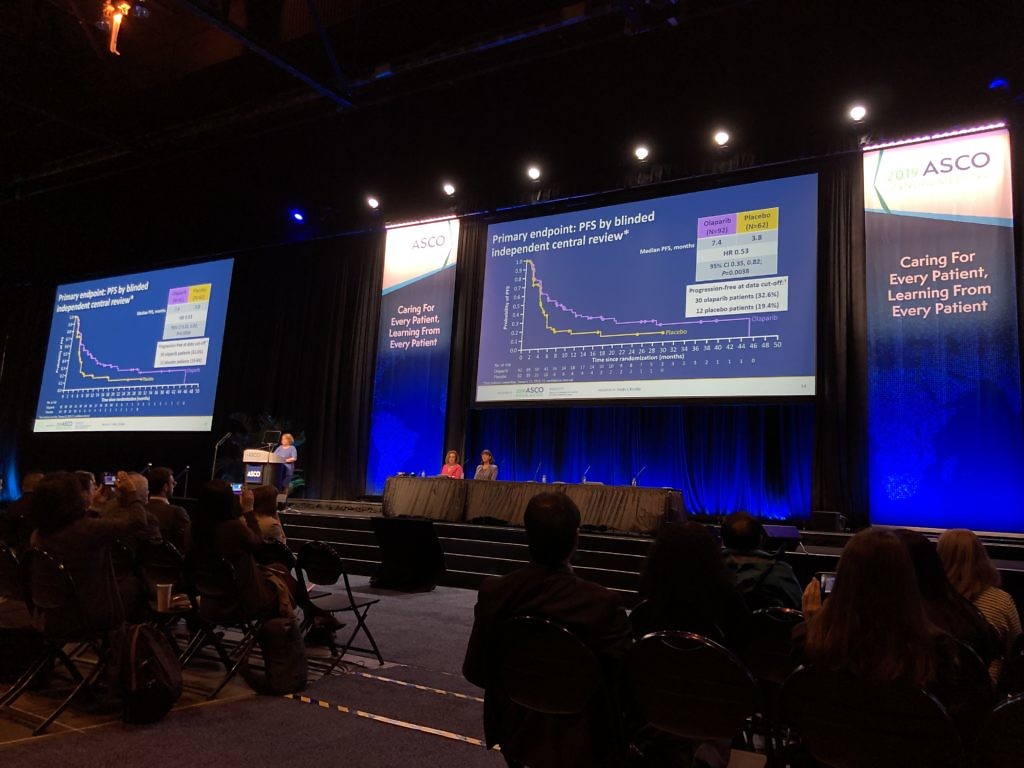

 The 2019 annual meeting of the American Society of Clinical Oncology (Twitter #ASCO19) is now in full swing, and we’re kicking off our on-site meeting coverage with a review of the some of the highlights of Friday here in Chicago.
The 2019 annual meeting of the American Society of Clinical Oncology (Twitter #ASCO19) is now in full swing, and we’re kicking off our on-site meeting coverage with a review of the some of the highlights of Friday here in Chicago.